- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

Open Chemistry Journal
(Discontinued)
ISSN: 1874-8422 ― Volume 8, 2021
Principle, Instrumentation, and Applications of UPLC: A Novel Technique of Liquid Chromatography
Gita Chawla*, Chanda Ranjan
Abstract
The key focus of the pharmaceutical or chemical industries is to reduce the cost involved in the development of new drugs and to improve the selectivity, sensitivity, and resolution for their detection. The purpose can now be solved by the separation method called UPLC which is the modified HPLC method comprising high pressure and small sized particles (less than 2 µm) used in the column, so the length of the column decreases leading to time saving and reduction in the consumption of solvent. The underlying principle of UPLC is based on van Deemter statement which describes the connection between linear velocity with plate height. UPLC contributes to the improvement of the three areas: speed, resolution, and sensitivity. This is a new advanced category of the HPLC which has the same basic principle and methodology with improved chromatographic performance. This review is an effort to compile the principle, instrumentation, and applications of UPLC.
Article Information
Identifiers and Pagination:
Year: 2016Volume: 3
First Page: 1
Last Page: 16
Publisher Id: CHEM-3-1
DOI: 10.2174/1874842201603010001
Article History:
Received Date: 03/04/2015Revision Received Date: 03/03/2016
Acceptance Date: 09/03/2016
Electronic publication date: 06/05/2016
Collection year: 2016
open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution-Non-Commercial 4.0 International Public License (CC BY-NC 4.0) (https://creativecommons.org/licenses/by-nc/4.0/legalcode), which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
* Address correspondence to this author at the Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Jamia Hamdard (Hamdard University), Hamdard Nagar, New Delhi-110 062, India; E-mail: gchawla@jamiahamdard.ac.in
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 03-04-2015 |
Original Manuscript | Principle, Instrumentation, and Applications of UPLC: A Novel Technique of Liquid Chromatography | |
INTRODUCTION
High-performance liquid chromatography (HPLC) is an important liquid chromatography (LC) technique used for the segregation of different components in mixtures. It is also used for the identification and quantification of compounds in the process of drug development and has been used over the world since decades. To further achieve the dramatic increase in resolution, speed and sensitivity in LC, a significant advancement in the instrumentation and column technology (column particle size and column dimension) were made [1Quanyun, A.X. Ultra-High Performance Liquid Chromatography and Its Applications; John Wiley & Sons: New Jersey, 2013. ]. To achieve the above targets, Waters in 2004, launched and trademarked Ultra Performance Liquid Chromatography (UPLC) which is based upon small, porous particles (sub 2micron particles). Van Deemter equation is the principle behind this evolution which correlates the connection between linear velocity and plate height. The small particles require a high pressure to work with UPLC i.e., 6000 psi which is typically the upper limit of conventional HPLCs. It was observed that when the particle size is decreased below 2.5 µm, there is a remarkable increase in the effectiveness and this effectiveness does not lessen on increasing the linear speed or rate of flow [2Chesnut, S.M.; Salisbury, J.J. The role of UHPLC in pharmaceutical development. J. Sep. Sci., 2007, 30(8), 1183-1190.
[http://dx.doi.org/10.1002/jssc.200600505] [PMID: 17595953] ]. The use of speed, particles with a small radius and maximum number of resolvable peaks (peak capacity) comprehends the efficiency together with resolution. This method reduces the mobile phase volume consumption by at least 80% compared to HPLC with a shorter runtime of about 1.5 min. The smaller sized particles increase the pressure up to 1000 bars or more which can alone increase the retention factor of the separation. Lower injection volume is required for UPLC which results in higher efficiency and increase in resolution. The higher column temperature reduces the mobile phase viscosity resulting in the high diffusion coefficient and flow rate without significant loss in efficiency and increase in column back pressure. UPLC is a special version of HPLC which has the advantage of technological strides made in particle chemistry performance, system optimization, detector design, data processing and control [3Fallas, M.M.; Neue, U.D.; Hadley, M.R.; McCalley, D.V. Further investigations of the effect of pressure on retention in ultra-high-pressure liquid chromatography. J. Chromatogr. A, 2010, 1217(3), 276-284.
[http://dx.doi.org/10.1016/j.chroma.2009.11.041] [PMID: 20015498] ]. These achievements lead to a very significant increase in resolution, sensitivity and efficiency with faster results and less consumption of solvents which lowers the cost and make the technology environment friendly also [4Nguyen, D.T.; Guillarme, D.; Heinisch, S.; Barrioulet, M.P.; Rocca, J.L.; Rudaz, S.; Veuthey, J.L. High throughput liquid chromatography with sub-2 microm particles at high pressure and high temperature. J. Chromatogr. A, 2007, 1167(1), 76-84.
[http://dx.doi.org/10.1016/j.chroma.2007.08.032] [PMID: 17765255] , 5LCGC: Solution for Seperation Scientist.
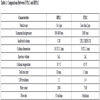
Available from: http://www.chromatographyonline.com]. Table 1 shows the comparison between UPLC and HPLC.
PRINCIPLE
The underlying principle of UPLC is based on the van Deemter relationship which explains the correlation between flow rate and plate height [5LCGC: Solution for Seperation Scientist.
Available from: http://www.chromatographyonline.com]. The van Deemter equation (i) shows that the flow range with the smaller particles is much greater in comparison with larger particles for good results [6Nguyen, D.T.; Guillarme, D.; Rudaz, S.; Veuthey, J.L. Fast analysis in liquid chromatography using small particle size and high pressure. J. Sep. Sci., 2006, 29(12), 1836-1848.
[http://dx.doi.org/10.1002/jssc.200600189] [PMID: 16970187] -9MacNair, J.E.; Lewis, K.C.; Jorgenson, J.W. Ultrahigh-pressure reversed-phase liquid chromatography in packed capillary columns. Anal. Chem., 1997, 69(6), 983-989.
[http://dx.doi.org/10.1021/ac961094r] [PMID: 9075400] ].
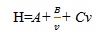 |
(i) |
Where H represents height equivalent to the theoretical plate (HETP), A, B & C are the constants and v is the flow rate (linear velocity) of the carrier gas. The aim is to minimize HETP to improve column efficiency. The term A does not depend on velocity and indicates eddy mixing. It is smaller if the columns are filled with small and uniform sized particles. The term B denotes the tendency of natural diffusion of the particles. At high flow rates, this effect is smaller, so this term is divided by v. The term C represents the kinetic resistance to equilibrium during the process of separation. The kinetic resistance is the time lag involved in moving from the mobile phase to the stationary phase and back again. The higher the flow rate of the mobile phase, the more a molecule on the packing material inclines to lag behind molecules in the mobile phase. Thus, this term is inversely proportional to linear velocity. Consequently, it is likely to enhance the throughput, and without affecting the chromatographic performance, the separation can be speeded up. The emergence of UPLC has necessitated the improvement of existing instrumentation facility for LC, which takes the benefit of the separation performance (by decreasing dead volumes) and consistent pressures (about 500 to 1000 bars, compared with 170 to 350 bars in HPLC). Efficiency is proportionate to the length of the column and inversely proportional to the radius of the particles [9MacNair, J.E.; Lewis, K.C.; Jorgenson, J.W. Ultrahigh-pressure reversed-phase liquid chromatography in packed capillary columns. Anal. Chem., 1997, 69(6), 983-989.
[http://dx.doi.org/10.1021/ac961094r] [PMID: 9075400] ]. Consequently, the column length can be reduced by the similar factor as the particle radius without affecting the resolution. The use of UPLC has helped in the detection of drug metabolites and enhancement of the quality of separation spectra [10Beattie, K.; Joncour, J.S.; Lawson, , K. Ultra performance liquid chromatography coupled to orthogonal quadrupole TOF-MS (MS) for metabolite identification. LC GC North America, 2005, 22-30., 11Wang, W.; Wang, S.; Tan, S.; Wen, M.; Qian, Y.; Zeng, X.; Guo, Y.; Yu, C. Detection of urine metabolites in polycystic ovary syndrome by UPLC triple-TOF-MS Clin. Chim.Acta, 2015, 448, 39-47.].
CHEMISTRY OF SMALL SIZE PARTICLES [12Wu, N.; Lippert, J.A.; Lee, M.L. Practical aspects of ultrahigh pressure capillary liquid chromatography. J. Chromatogr. A., 2001, 911(1), 1-12.
[http://dx.doi.org/10.1016/S0021-9673(00)01188-2] [PMID: 11269586] -14Dong, M.W. Modern HPLC for Practicing Scientists; Wiley Interscience: New Jersey, 2006, pp. 35-37.
[http://dx.doi.org/10.1002/0471973106] ]
The chemistry of the particles used in this course of the method contributes the increased efficiency and potential to work at amplified linear velocity, thereby, providing both the speed and the resolution. Efficiency is one of the important separation parameters which plays a significant role in UPLC since it depends on the same selectivity and retentivity as HPLC. This may be understood with the help of the following basic resolution (Rs) equation (ii):
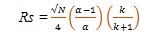 |
(ii) |
Where α represents the selectivity factor, N is efficiency and K denotes proportionality constant. According to this, resolution increases with increase in efficiency. Since efficiency (N) is inversely proportional to particle size (dp) equation (iii):
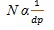 |
(iii) |
As the size of the particle decreases by a factor of three, there is three times increase in the efficiency and in the resolution, there is an increase of the square root of three (nine times). So, there is an increase in efficiency with consequent increase in resolution and sensitivity as the particle size decreases. Similarly, efficiency (N) is directly proportional to the length of the column (L). So, the equation (iii) may be written as equation (iv) as follows:
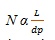 |
(iv) |
Therefore, the length of the column (L) may be reduced by the same ratio as the size of the particle without loosing the resolution.
Efficiency also has indirect relationship with the peak width (w) according to the equation (v):
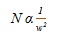 |
(v) |
This means that narrower the peaks are, easier is their separation from each other. Peak height (H) also has opposite relationship with peak width (w) according to equation (vi):
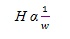 |
(vi) |
Based on the above facts, in UPLC, by reducing the particle size (1/3rd), the column length is reduced (1/3rd), the flow rate is increased (3 times) and the separation is done faster (1/9th time) with maintaining the resolution [12Wu, N.; Lippert, J.A.; Lee, M.L. Practical aspects of ultrahigh pressure capillary liquid chromatography. J. Chromatogr. A., 2001, 911(1), 1-12.
[http://dx.doi.org/10.1016/S0021-9673(00)01188-2] [PMID: 11269586] ]. For this reason, short columns packed with small size particles (about 2 μm) are used with these systems, to quicken the separation with higher efficiency, while maintaining a tolerable loss of load. The effect of particle size on HETP and linear velocity has been illustrated in Fig. (1 ). by using van Deemter plot. Fig. (1
). by using van Deemter plot. Fig. (1 ) shows that the particles with a smaller diameter are contributing less to band broadening compared to larger particles and are less affected by higher column flow rate [15Van Deemter, J.J.; Zuiderweg, E.J.; Klinkenberg, A. Longitudinal diffusion and resistance to mass transfer as causes of non ideality in chromatography. Chem. Eng. Sci., 1956, 5, 271-289.
) shows that the particles with a smaller diameter are contributing less to band broadening compared to larger particles and are less affected by higher column flow rate [15Van Deemter, J.J.; Zuiderweg, E.J.; Klinkenberg, A. Longitudinal diffusion and resistance to mass transfer as causes of non ideality in chromatography. Chem. Eng. Sci., 1956, 5, 271-289.
[http://dx.doi.org/10.1016/0009-2509(56)80003-1] , 16David, G.W. Pharmaceutical analysis. Sec edition; Elsevier Churchill Livingstone: UK, 2005, pp. 224-225.].
 |
Fig. (1) Van Deemter Plot illustrating the effect of particle size (in µm) on plate height (H). Smaller particle size provides higher overall peak efficiencies and a much wide range of flow rates. |
ADVANTAGES OF UPLC [17Roge, A.B.; Firke, S.N.; Dhane, R.M.; Gunjkar, V.J.; Vadvalkar, S.M. Novel achievement of HPLC: UPLC. Int. J. Pharm. Tech. Res., 2011, 3(3), 1423-1429.- 19Nováková, L.; Solichová, D.; Solich, P. Advantages of ultra performance liquid chromatography over high-performance liquid chromatography: comparison of different analytical approaches during analysis of diclofenac gel. J. Sep. Sci., 2006, 29(16), 2433-2443.
[http://dx.doi.org/10.1002/jssc.200600147] [PMID: 17154123] ]
It is more selective and sensitive with high resolution performance and faster resolving power. It also reduces process cycle time and assures end-product quality with reduced cost of operation and decreased run time. It increases sensitivity and provides quick analysis through the use of a novel column material of very small particle size. It decreases the consumption of solvent and increases sample throughput and also provides real-time analysis in step with manufacturing processes.
DISADVANTAGES [20Wu, N.; Lippert, J.A.; Lee, M.L. Practical aspects of ultrahigh pressure capillary liquid chromatography. J. Chromatogr. A., 2001, 911(1), 1-12.
[http://dx.doi.org/10.1016/S0021-9673(00)01188-2] [PMID: 11269586] - 22Gaikwad, P.V. Ultra Performance Liquid Chromatography: A recent novel development in HPLC. Inter. J. of Compre. Pharm., 2010, 2(08), 1-3.]
A major disadvantage of UPLC is the higher back pressures compared to conventional HPLC which decreases the life of the columns. Increasing the column temperature reduces the back pressure problem in UPLC. Moreover, the particles of less than 2 μm are mostly non-regenerable and, therefore, have a narrow use.
INSTRUMENTATION
The instrumentation of UPLC includes- sample injection, UPLC columns and detectors.
Sample Injection
The use of the injector is to add precisely measured, a small volume of solution containing the sample in the mobile phase. The injection must be done reproducibly and accurately. Conventional injection valves may be manual or programmed and to guard the column from extreme pressure instabilities, the injection process must be comparatively pulse-free. To reduce the potential band spreading, the swept volume of the device is desired to be minimal. A quick injection cycle time is required to fully avail the speed afforded by UPLC. To increase the sensitivity, low volume injections with minimal carryover are required [23Prathap, G.M.; Nishat, A. Ultra performance liquid chromatography: A chromatography technique. Int. J. Pharmacy, 2013, 3(1), 251-260.]. The volume of the sample in UPLC is usually 2-5 μL. Nowadays, direct injection approaches are utilized for the biological samples [24Lars, Y.; Honore, H.S. On-line turbulent-flow chromatography-high-performance liquid chromatography-mass spectrometry for fast sample preparation and quantitation. J. Chromatogr, 2003, A 1020, 59-67.- 26Broske, A.D. Agilent technologies application note, 2004. Available at: https://www.agilent.com/cs/library/primers/Public/5991-3326EN_SPHB.pdf].
UPLC Column
Columns used for UPLC have been developed and manufactured by the following different companies:
- Waters: Acquity UPLC columns and Vanguard Pre-columns have been produced [27Acquity UPLC column. Available at: https://www.waters.com/webassets/cms/library/docs/720001140en.pdf, ].
- Agilgent technology provides highest performing columns that provide fast and reproducible results. These include Poroshell 120 columns, ZORBAX Rapid Resolution High definition columns, ZORBAX Eclipse plus columns and ZORBAX Rapid Reduction High Throughput columns [28Agilent Technologies. Available at: https://www.agilent.com/cs/library/catalogs/public/5991-1059EN%20LC_Columns.pdf, ].
- Altech Assosciate [29Global Manufacturer pages. Available at: http://http://www.mfgpages.com/, ].
- Phenomenex provides Kinetex® Coreshell HPLC/UHPLC columns of high efficiency and performance [30Phenomenex. Available at: http://http://www.phenomenex.com/, ].
Different types of columns being used in UPLC are packed with particles which are produced through different technologies [27Acquity UPLC column. Available at: https://www.waters.com/webassets/cms/library/docs/720001140en.pdf, ]. These are as follows:
- Charged Surface Hybrid [CSH] particle technology,
- Ethylene Bridged Hybrid [BEH] particle technology,
- High Strength Silica [HSS] particle technology and
- Peptide Separation Technology (PST).
CSH Particle Technology [27Acquity UPLC column. Available at: https://www.waters.com/webassets/cms/library/docs/720001140en.pdf, ]
CSH Technology is the newest methodology in the development of hybrid materials which utilizes low-level surface charged particles for the enhancement of the selectivity and sharpness of the peaks. Hybrid based packing material approach provides sharp peaks specially for basic compounds under low pH with higher efficiency and chemical stability. CSH C18, CSH Phenyl hexyl, and CSH Fluoro phenyl are the different types of CSH particles being widely used.
These columns have the advantage of exceptional peak shape, increased loading capacity (CSH C18); complementary selectivity to straight chain alkyl phases (CSH-phenyl-hexyl); selectivity for positional isomers, halogenated and polar compounds (CSH-fluoro phenyl). The other advantages include- higher stability at a wide range of pH, improved batch to batch reproducibility and fast column equilibration after any change in the pH of the mobile phase.
Applications of CSH technology based columns include the analysis of basic compounds even in their ionized form. While analyzing the basic compounds under low pH and reversed phase conditions, poor peak shape and retention often result. Whereas, CSH Phenyl hexyl columns provide exceptional peak shape for basic drugs under acidic mobile phase conditions. Fig. (2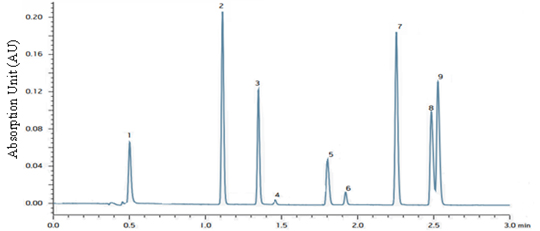 ) demonstrates the analysis of basic drugs using CSH technology based columns.
) demonstrates the analysis of basic drugs using CSH technology based columns.
BEH Particle Technology [27Acquity UPLC column. Available at: https://www.waters.com/webassets/cms/library/docs/720001140en.pdf, ]
For more than a decade, hybrid particle technology [HPT] has delivered incomparable versatility and performance, enabling chromatographers to push the limits of LC separations. The XTerra particle was the first commercially available option to improve the issues (poor peak shape for basic compounds and column longevity due to chemical instability) without the drawbacks of unpredictable selectivity produced by alternative materials such as zirconia, organic polymers, and graphitic carbon. With the commercialization of 2.5 μm XTerra particles, the concept of fast HPLC with small particles was born, improving the productivity of chromatographic laboratories globally.
Straight chain alkyl columns (BEH C18 and C8), Embedded polar group column (BEH Shield RP18) and UPLC BEH Phenyl (phenyl group tethered to the silyl functionality with a C6 alkyl) are the different types of BEH particle technology based columns which are being widely used.
These columns provide higher retention, selectivity (C18); exceptional efficiency, peak symmetry, chemical stability (C8); enhanced peak shape and higher compatibility with 100% aqueous mobile phase (RP18); chemical stability, reproducibility and peak shape (phenyl). Applications include the rapid assay of cytochrome p450 isoenzymes which are responsible for more than 90% of drug metabolism on the market today. Cytochrome p450 isoenzymes are used to determine the level of drug inhibition, induction or drug-drug interaction that takes place. The ultra-low dispersion and system dwell volume of UPLC technology enables the rapid analysis of these enzymes in less than 30 seconds. (Fig. 3 ). shows the chromatogram of an assay of cytochrome P450.
). shows the chromatogram of an assay of cytochrome P450.
HSS Particle Technology [27Acquity UPLC column. Available at: https://www.waters.com/webassets/cms/library/docs/720001140en.pdf, ]
HSS particle technology is the advanced technology, born from an innovative synthetic process in which the mechanical stability is improved while the pore volumes remain similar to that of HPLC silica-based materials. This results in an advanced technique which provides higher retention in comparison to the hybrid particles. HSS T3, HSS C18, HSS C18 SB, HSS PFP and HSS CN are the different types of HSS particles being widely used.
These columns provide balanced retention of polar and hydrophobic molecule (T3); exceptional peak shapes, increased retention (C18); greater retention of basic compounds (CS 18 SB); ideally suited for planar aromatic, positional, halogenated compounds (PFP); ultra stable retention and compatible with both reversed-phase and normal-phase techniques (CN).
Applications of this technology include the analysis of tetracycline antibiotics which are commonly prescribed for the treatment of bacterial infections. The ACQUITY UPLC HSS C8 column enables a single UV-based method for the simultaneous separation of oxytetracycline including its degraded and related products (which are produced due to drug fermentation)as well as additional veterinary antibiotics (Fig. 4 ).
).
PST Columns [27Acquity UPLC column. Available at: https://www.waters.com/webassets/cms/library/docs/720001140en.pdf, ]
PST utilizes the C18 BEH Technology TM particles whose particles sizes range from 1.7 µm to 10 µm and the column dimension ranges from 75 µm to 30 mm internal diameter (i.d) and column length from 50 mm to 250 mm. They are used in all kind of research and development that involves analysis and isolation of peptides. The PST columns provide sharp symmetrical peaks.
Solvent Delivery System
The solvent delivery system must perform reproducible high pressure pumping with a smooth and constant flow of solvents. UPLC systems routinely operate at 8000-15000 psi. The delivery system must also remunerate for a variety of solvents used in isocratic, linear & nonlinear gradient elution and solvent compressibility for a wide range of pressures. The Acquity UPLC binary solvent manager has two solvent delivery modules operating in parallel for high pressure merging of two solvents in <140 μL internal system volume. The dissolved gases are removed by vacuum up to four eluents plus two wash solvents [24Lars, Y.; Honore, H.S. On-line turbulent-flow chromatography-high-performance liquid chromatography-mass spectrometry for fast sample preparation and quantitation. J. Chromatogr, 2003, A 1020, 59-67., 26Broske, A.D. Agilent technologies application note, 2004. Available at: https://www.agilent.com/cs/library/primers/Public/5991-3326EN_SPHB.pdf].
The Detector
The detector employed for the UPLC should be able to give a high sampling rate with narrow obtainable peaks (<1 s half-height peak width) and the dispersion of the peaks should be minimum so that the wastage of the separated solute is less on the column. The UPLC technique provides the sensitivity of separation two to three times more than the previous analytical method HPLC, which is also due to the method employed for the detection. The detectors employed in the UPLC are Acquity photodiode array (PDA) and Tunable Vis-UV (TUV) in which Teflon AF is used which provides an internally reflective surface and enhances the light transmission efficiency by eliminating the internal absorptions. These have path lengths 10 nun, acquisition rates 20 (PDA) and 40 (TUV) points, and total internal volume 500 nL. Mass spectrometric detection has also been used with UPLC [24Lars, Y.; Honore, H.S. On-line turbulent-flow chromatography-high-performance liquid chromatography-mass spectrometry for fast sample preparation and quantitation. J. Chromatogr, 2003, A 1020, 59-67., 26Broske, A.D. Agilent technologies application note, 2004. Available at: https://www.agilent.com/cs/library/primers/Public/5991-3326EN_SPHB.pdf].
Applications of UPLC
Determination of Pesticides in Groundwater [31Mezcua, M.; Agüera, A.; Lliberia, J.L.; Cortés, M.A.; Bagó, B.; Fernández-Alba, A.R. Application of ultra performance liquid chromatography-tandem mass spectrometry to the analysis of priority pesticides in groundwater. J. Chromatogr. A, 2006, 1109(2), 222-227.
[http://dx.doi.org/10.1016/j.chroma.2006.01.024] [PMID: 16451801] ]
UPLC coupled with triple quadrupole tandem mass spectrometry (UPLCTM-MS/MS) can be utilized to determine the trace level pesticides in groundwater in less time and speedy manner. The technique has enhanced the analysis speed, sensitivity, and resolution.
Improved Resolving Power in Peptide Maps [27Acquity UPLC column.
Available at: https://www.waters.com/webassets/cms/library/docs/720001140en.pdf, , 32Lauber, M.A.; Koza, S.M.; McCall, S.A.; Alden, B.A.; Iraneta, P.C.; Fountain, K.J. High-resolution peptide mapping separations with MS-friendly mobile phases and charge-surface-modified C18. Anal. Chem., 2013, 85(14), 6936-6944.
[http://dx.doi.org/10.1021/ac401481z] [PMID: 23772755] ]
Peptide mapping is an essential technique for the characterization of proteins. Due to exceptionally reduced instrument and column dispersion, the analyzes of tryptic digest of phosphorylase by UPLC technology provides significantly improved resolution, peak capacity, and sensitivity compared to HPLC, allowing the detailed characterization of the protein (Fig. 5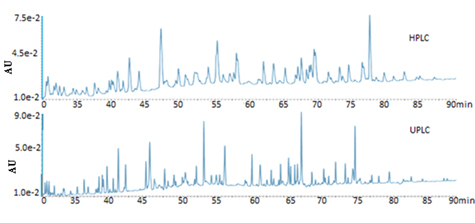 ).
).
 |
Fig. (5) Improved resolving power in peptide maps by using UPLC. |
Rapid Dose Formulation Analysis [33Salane, K.; Stoffolano, P.J.; Robinson, E.; Eichhold, T. F; Hoke, I. S.; Baker, H.; Richardson, T. R.; Wehmeyer, E. C.; Kenneth, R. The evaluation and application of UPLC for the rapid analysis of dose formulations. LC GC, 2005, 23(Suppl.), 36., 34Salane, K.; Peter, J.; Eric, R.; Thomas, E.; Steven, H.; Timothy, R.; Eloise, C.; Kenneth, R. The evaluation and application of UPLC for the rapid analysis of dose formulations. Sep. Sci. Red., 2005, 23(5), 36-39.]
Nowadays, the use of UPLC together with UV and MS detection has been widely utilized in pharmaceutical applications. Several commercial drug formulations were used as models to study the efficiency of separations with the change of flow rate. The efficiency was judged on the parameters of resolution, theoretical plates, column ruggedness, retention time, and peak area. For example, mefenamic acid and chloramphenicol separation was studied in dimethylacetamide/ polyethylene glycol-200 vehicle.
Analysis of Traditional Chinese Medicines (TCM) [35Yang, G.L.; Yang, L.W.; Li, Y.X.; Cao, H.; Zhou, W.L.; Fang, Z.J.; Zhou, H.B.; Mo, J.L.; Xiao, S.X.; Lin, H.R. Applications of ultra-performance liquid chromatography to traditional Chinese medicines. J. Chromatogr. Sci., 2010, 48(1), 18-21.
[http://dx.doi.org/10.1093/chromsci/48.1.18] [PMID: 20056030] ]
The identification and quantification of components of TCM by chromatographic analysis is one of the major challenges. TCM is a complex matrix in which all the constituents play a specific role for the overall efficacy. Therefore, the analysis of all the constituents is synchronously necessary for the quality control. The new technique UPLC is used for the quality control of the TCM.
Multi-Residue Analysis of Pharmaceuticals in Waste Water [36Petrovic, M.; Gros, M.; Barcelo, D. Multi-residue analysis of pharmaceuticals in wastewater by ultra-performance liquid chromatography-quadrupole-time-of-flight mass spectrometry. J. Chromatogr. A, 2006, 1124(1-2), 68-81.
[http://dx.doi.org/10.1016/j.chroma.2006.05.024] [PMID: 16759662] ]
The water used in the pharmaceutical companies is found to have the traces of various cholesterol-lowering statin agents, anti-ulcer agents, antibiotics, beta-blockers, analgesics, anti-inflammatory agents, lipid regulating agents, psychiatric drugs, and histamine H2 receptor antagonists. UPLC coupled with Q-TOF-MS is used to confirm and screen these drugs in the samples of waste water treatment plant.
Identification of Metabolites [37Mao, J.; Xu, Y.; Lu, B.; Liu, J.; Hong, G.; Zhang, Q.; Sun, S.; Zhang, J. Simultaneous determination of nicotine and its nine metabolites in rat blood utilizing microdialysis coupled with UPLC-tandem mass spectrometry for pharmacokinetic application. Anal. Bioanal. Chem., 2015, 407(14), 4101-4109.
[http://dx.doi.org/10.1007/s00216-015-8643-0] [PMID: 25824453] - 39Zeng, W.; Han, H.; Tao, Y.; Yang, L.; Wang, Z.; Chen, K. Identification of bio-active metabolites of gentiopicroside by UPLC/Q-TOF MS and NMR. Biomed. Chromatogr., 2013, 27(9), 1129-1136.
[http://dx.doi.org/10.1002/bmc.2917] [PMID: 23733682] ]
The identification and detection of all the possible metabolites of the candidate drugs for the discovery of new chemical entities is a very important step. For the identification of the metabolites, a high sample throughput is required to be maintained by the analysts to provide quick results to the medicinal chemists. UPLC-MS/MS is helpful in biomarker discovery as it meets tough analytical requirements and provides sensitivity, mass accuracy, dynamic range, and resolution.
In Manufacturing / Quality Assurance (QA) / Quality Control (QC) [40Dhekale, N.H.; Bindu, K.H.; Kirankumar, K.Y.; Gore, A.H.; Anbhule, P.V.; Kolekar, G.B. Development and optimization of a multivariate RP-UPLC method for determination of telmisartan and its related substances by applying a two-level factorial design approach: Application to quality control study. Anal. Methods, 2014, 6(14), 5168-5182.
[http://dx.doi.org/10.1039/c3ay42260g] ]
Identification, quantification, purification, efficacy and safety are key parameters to be evaluated during manufacturing of a drug product and pharmaceutical dosage form. Material stability is also observed as a component of QA and QC. UPLC is used as an important tool in QA/QC laboratories for the quantitative and extremely regulated analysis.
Impurity Profiling [41Srivastava, B.; Sharma, B.K.; Baghel, U.S.; Yashwant, S.N. Ultra performance liquid chromatography: A chromatography technique. Int. J. Pharm. Qua. Assur., 2010, 2(1), 19-25.- 43Prakash, L.; Malipeddi, H.; Subbaiah, B.V.; Lakka, N.S. Impurity profiling and a stability-indicating UPLC method development and validation for the estimation of related impurities of halobetasol propionate in halobetasol propionate 0.05% (w/w) cream. J. Chromatogr. Sci., 2015, 53(1), 112-121.
[http://dx.doi.org/10.1093/chromsci/bmu027] [PMID: 24795078] ]
Impurity profiling should be efficient for consistent detection and separation of all the impurities present in the active compound. The drug development and formulation process demand accurate measurement/testing of low-level impurities present with the active pharmaceutical ingredients or the excipients or the raw materials used in the preparation of the final product. Thus, the presence of excipients in the sample makes the profiling difficult and with HPLC method, it takes longer time for analysis to achieve sufficient resolution. Thus, the combination of UPLC with mass spectrometry has been useful for the documentation of drug and endogenous metabolites in the final product.
Method Development / Validation [41Srivastava, B.; Sharma, B.K.; Baghel, U.S.; Yashwant, S.N. Ultra performance liquid chromatography: A chromatography technique. Int. J. Pharm. Qua. Assur., 2010, 2(1), 19-25., 44Shaligram, S.R.; Ajameri, A.; Mody, R.; Padmaja, P. Development and validation of RP-HPLC and RP-UPLC methods for quantification of erythropoietin formulated with human serum albumin. J. Pharm. Anal., 2012, 2(2), 160-165.
[http://dx.doi.org/10.1016/j.jpha.2011.11.006] , 45Ram, V.; Kher, G.; Dubal, K.; Dodiya, B.; Joshi, H. Development and validation of a stability indicating UPLC method for determination of ticlopidine hydrochloride in its tablet formulation. Saudi Pharm. J., 2011, 19(3), 159-164.
[http://dx.doi.org/10.1016/j.jsps.2011.03.005] [PMID: 23960754] ]
Method development and validation is a complex process and consumes a lot of time. For the development of a robust and reliable method, the labs are required to study many combinations of different parameters e.g. mobile phase, temperature, pH, column and gradient chemistry etc. UPLC is an important method used in the laboratory which reduces the cost and increases the efficiency of analysis required for developing and validating the method. With UPLC, the speed of the separation increases and efficiency improves, which results in the fast development of methodologies. High stability of the UPLC columns provides the possibility of selection of column temperature and pH from a wide range.
Forced Degradation Studies (FDS) [27Acquity UPLC column.
Available at: https://www.waters.com/webassets/cms/library/docs/720001140en.pdf, , 46Pradipbhai, D.K.; Sharma, M.; Garg, P.; Thota, J.R.; Ragampeta, S.; Talluri, M.V. Characterization of stress degradation products of mirabegron using UPLC-QTOF-MS/MS and in silico toxicity predictions of its degradation products. RSC Adv., 2015, 5, 31024-31038.
[http://dx.doi.org/10.1039/C5RA01711D] ]
This study is done to access the chemical stability of the candidate compound in the pharmaceuticals. Usually, it is performed at the preliminary stage in the process of drug development. Forced degradation/ stress testing is performed under accelerated environment. The experimental conditions cause the candidate compound to degrade under extreme conditions like acid and base hydrolysis, peroxide oxidation, photo-oxidation and thermal stability to identify the resultant degradation products. This helps to establish degradation pathways and thus intrinsic stability of a drug substance. The stability of product describes shelf life and storage conditions and helps in the selection of appropriate formulations and their suitable packaging. This is compulsory for regulatory documentation. The commonly used analytical approach for FDS is HPLC with UV and/ or MS but these techniques consume a lot of time and not provide high resolution to confirm the precise detection of degradation products. Use of UPLC with photodiode array and MS analysis supports the identification of degradation products and also reduces the time needed to evolve stability indicating methods. Fig. (6 ) is a chromatogram of FDS of glimepiride done on UPLC BEH C18 column. It shows the sharp peaks of different degradation products along with glimepiride.
) is a chromatogram of FDS of glimepiride done on UPLC BEH C18 column. It shows the sharp peaks of different degradation products along with glimepiride.
 |
Fig. (6) Chromatogram of high resolution analysis of Glimepiride forced degradation on BEH C18. |
Dissolution Testing [47Karunakaran, K.; Navaneethan, G.; Elango, K.P. Development of a new RP-UPLC method for the determination of rabeprazole sodium in pharmaceutical formulation and application in dissolution studies. Trop. J. Pharm. Res., 2011, 10(5), 655.]
Dissolution testing is one of the most important step carried out during formulation and manufacturing process to test the drug release. The dissolution data provides understanding to validate consistency and uniformity of the active ingredient in every batch. Testing of potent drugs in sustained release dosage form is very important as their dissolution studies data can affect the delivery of the medicine. Moreover, new and potent formulations require higher separation sensitivity. UPLC method provides accurate and consistent automated online sample acquirement.
Bioequivalence / Bioanalysis Studies [48Yadav, M.; Rao, R.; Kurani, H.; Singhal, P.; Goswami, S.; Shrivastav, P.S. Application of a rapid and selective method for the simultaneous determination of protease inhibitors, lopinavir and ritonavir in human plasma by UPLC-ESI-MS/MS for bioequivalence study in Indian subjects. J. Pharm. Biomed. Anal., 2009, 49(4), 1115-1122.
[http://dx.doi.org/10.1016/j.jpba.2009.02.010] [PMID: 19282124] , 49Pidpruzhnykov, Y.V.; Sabko, V.E.; Iurchenko, V.V.; Zupanets, I.A. UPLC-MS/MS method for bioequivalence study of oral drugs of meldonium. Biomed. Chromatogr., 2012, 26(5), 599-605.
[http://dx.doi.org/10.1002/bmc.1703] [PMID: 21915891] ]
Bioequivalence studies are pharmacokinetic studies needed for the quantitation of drugs in biological samples. This is an important step to compare the rate and exposure level of newly developed formulations of prevailing drugs with that of the original formulation. The selectivity and sensitivity of UPLC-MS/MS produce reliable and precise data. UPLC- MS/MS solutions have increased efficacy, output, and profitability for the bioequivalence laboratories. UPLC sample manager enhances the effectiveness by considering a huge number of samples in a temperature controlled atmosphere, confirming maximum throughput which increases the sensitivity and quality of data acquisition rates of tandem quadrupole MS systems.
Toxicity Studies [50Zhang, S.; Cheng, X.; Wang, Y.; Fan, J.; Li, R.; Zhou, S.; Liu, S.; Shi, J.; Sun, J.; Hu, Y.; Xu, C.; Wu, C.; Chang, X.; Tang, L.; Zhou, Z. Ninety day toxicity and toxicokinetics of fluorochloridone after oral administration in rats. Int. J. Environ. Res. Public Health, 2015, 12(5), 4942-4966.
[http://dx.doi.org/10.3390/ijerph120504942] [PMID: 25955529] ]
During the drug development process, toxicity issue causes a fall out of drug candidates and this causes monetary loss to the organization. It is a complicated task to estimate candidate drugs for possible inhibition or initiation of metabolizing enzymes, toxicity or drug-drug interactions in the body. UPLC allows precise detection due to its high resolution. Further, its sensitivity also allows the detection of the peaks at low concentrations. These factors lessen the time for analysis and decrease failure of sample analysis.
Iodinated Disinfection Byproducts (IDBPs) [51Ding, G.; Zhang, X. A picture of polar iodinated disinfection byproducts in drinking water by (UPLC/)ESI-tqMS. Environ. Sci. Technol., 2009, 43(24), 9287-9293.
[http://dx.doi.org/10.1021/es901821a] [PMID: 20000522] ]
Till date, a few numbers of IDBPs have been characterized in drinking water by using GC/MS. But with the help of coupling UPLC to the electrospray ionization-triple quadrupole mass spectrometer (ESI-tqMS), pictures of IDBPs in samples of water, treated with chlorine and chlorine- ammonia have been collected and 17 IDBPs structures were provisionally projected.
Therapeutic Drug Monitoring [52Carlier, M. Quantification of seven β- lactam antibiotics and two β- lactamase inhibitors in human plasma using a validated UPLC-MS/MS method. Int. J. Antimicrob. Agents, 2012, 40(5), 416-422., 53Thomas, T.; Petrie, K.; Shim, J.; Abildskov, K.M.; Westhoff, C.L.; Cremers, S. A UPLC-MS/MS method for therapeutic drug monitoring of etonogestrel. Ther. Drug Monit., 2013, 35(6), 844-848.
[http://dx.doi.org/10.1097/FTD.0b013e31829a10fa] [PMID: 24081205] ]
Carlier et al in 2012 reported the monitoring of β- lactam antibiotic concentration in plasma of patients with different pharmacokinetics. In their studies, they tried to validate a UPLC-MS/MS method for the simultaneous estimation of two β -lactamase inhibitors and seven β- lactam antibiotics in human plasma. The main benefit of the technique is the faster speed of analysis (5.5 min /sample) compared to other approaches used for this type of multiple analytes.
Analysis of Explosives [54Stuart, A. Analysis of explosives using Ultra Performance Liquid Chromatography with UV and/ or Mass Spectroscopy Detection. J. Ener. Mat., 2008, 26(4), 197-206.]
UPLC proved to be an enhanced procedure to analyze various explosives. In addition, analysis of explosive remains from hand swipes can also be detected by UPLC with minimal sample preparation requirement (less than 9 min), thus enhancing the lab output as well as freeing up valuable MS time for further analysis
Analysis of Contaminants in Foodstuffs [55Schummer, C.; Sassel, J.; Bonenberger, P.; Moris, G. Low-level detections of Sudan I, II, III and IV in spices and Chili-containing foodstuffs using UPLC-ESI-MS/MS. J. Agric. Food Chem., 2013, 61(9), 2284-2289.
[http://dx.doi.org/10.1021/jf400602a] [PMID: 23390927] ]
Sudan I-IV is a red colored dye which is commonly used in petrochemical industries. The intensive color of Sudan dyes lured frauds for improving the color of several spices and food stuffs which can form DNA adducts causing mutations. UPLC coupled to tandem mass spectrometry allows the identification of Sudan at low ppb levels in spices and chilli containing food stuffs. Fig. (7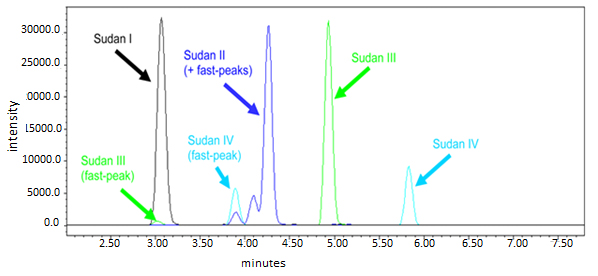 ) shows the chromatogram of Sudan I-IV and it also contains the fast peaks of sudan II, III and IV. These fast peaks are additional peaks as these eluted a few minutes before the main peak of the compounds.
) shows the chromatogram of Sudan I-IV and it also contains the fast peaks of sudan II, III and IV. These fast peaks are additional peaks as these eluted a few minutes before the main peak of the compounds.
 |
Fig. (7) Chromatogram of Sudan I – IV at 50 µg/L. |
Dendrimers Characterization [56Chevelle, A.C.; Stuart, A.O.; Thomas, A.F. Improved methodology for monitoring poly(amidoamine) dendrimers surface transformations and product quality by ultra performance liquid chromatography. J. Nanomat., 2008, 1-7.
[http://dx.doi.org/10.1155/2008/456082] , 57Biricova, V.; Laznickova, A. Dendrimers: Analytical characterization and applications. Bioorg. Chem., 2009, 37(6), 185-192.
[http://dx.doi.org/10.1016/j.bioorg.2009.07.006] [PMID: 19703699] ]
Dendrimers are highly branched symmetric polymers having a compact round structure (diameter 1.1nm to 9 nm) and unique behavior. They are normally synthesized from a central polyfunctional core by repetitive addition of polymers. Dendrimers surfaces provide a brilliant stage for the attachment and appearance of cell specific targeting groups, solubility modernizers and stalth moieties that decrease immunological interactions. Polyamidoamine (PAMAM) dendrimers are one of the widely used dendrimers. HPLC has been utilized to isolate and to check the purity of many PAMAM dendrimer generation or conjugates. This technique also helps to study the solubility of multi functionalized dendrimers and the interactions between them and biomolecules. UPLC reduces the retention time of analytes with an improvement of the resolution proficiency during dendrimers studies
Determination of Phytoconstituents [58Quanyun, A.X. Ultra-High Performance Liquid Chromatography and Its Applications; Wiley & Sons: New Jersey, 2013, pp. 197-234.-62Chen, X.J.; Ji, H.; Zhang, Q.W.; Tu, P.F.; Wang, Y.T.; Guo, B.L.; Li, S.P. A rapid method for simultaneous determination of 15 flavonoids in Epimedium using pressurized liquid extraction and ultra-performance liquid chromatography. J. Pharm. Biomed. Anal., 2008, 46(2), 226-235.
[http://dx.doi.org/10.1016/j.jpba.2007.09.016] [PMID: 17961954] ]
UPLC can be used to identify and quantify procyanadines, phenolic compounds, monomers, oligomers, isoflavones, flavonoids, coumarins and alkaloids such as caffeine and theobromine. Fig. (8 ) shows the comparison of chromatograms for analysis of coumarins using HPLC and UPLC methods. It is clear from this figure that UPLC method completes the process in less time and sharp peaks are obtained.
) shows the comparison of chromatograms for analysis of coumarins using HPLC and UPLC methods. It is clear from this figure that UPLC method completes the process in less time and sharp peaks are obtained.
 |
Fig. (8) Chromatogram of the Analysis of Coumarins by HPLC and UPLC. |
Identifying Static and Kinetic Lipid Phenotype [63Castro-Perez, J.M.; Roddy, T.P.; Shah, V.; McLaren, D.G.; Wang, S.P.; Jensen, K.; Vreeken, R.J.; Hankemeier, T.; Johns, D.G.; Previs, S.F.; Hubbard, B.K. Identifying static and kinetic lipid phenotypes by high resolution UPLC-MS: unraveling diet-induced changes in lipid homeostasis by coupling metabolomics and fluxomics. J. Proteome Res., 2011, 10(9), 4281-4290.
[http://dx.doi.org/10.1021/pr200480g] [PMID: 21744776] ]
High resolution UPLC-MS is employed to study the concentration of lipids and their endogenous production. Therefore, this technique was found to be useful in determining the contribution of different pathways and synthesis that could affect lipid biology.
Analysis of Free Amino Acids (FAA) in Wines [64Fiechte, G.; Mayer, H.K. UPLC analysis of free amino acids in wines: Profiling of on-leesaged wines. J. Chromato. B., 2010, 879, 1361-1366.
[http://dx.doi.org/10.1016/j.jchromb.2011.02.005] ]
The production of FAA in the less aged white wines can be determined by the UPLC. The UPLCTM method is an established method for the analysis of amino acids using 6-aminoquinolyl. This new UPLCTM method has made the separations quick and reliable for 24 amino acids within 23 minutes. This method proved to be superior compared to original HPLC method due to much improvement in resolution with reduced run time. Fig. (9 ) shows the contribution of UPLC in monitoring the complex autolysis processes during the on-lees aging of wines.
) shows the contribution of UPLC in monitoring the complex autolysis processes during the on-lees aging of wines.
Identification of Metabolic Biomarkers to Diagnose Epithelial Ovarian Cancer (EOC) [65Fan, L.; Zhang, W.; Yin, M.; Zhang, T.; Wu, X.; Zhang, H.; Sun, M.; Li, Z.; Hou, Y.; Zhou, X.; Lou, G.; Li, K. Identification of metabolic biomarkers to diagnose epithelial ovarian cancer using a UPLC/QTOF/MS platform. Acta Oncol., 2012, 51(4), 473-479.
[http://dx.doi.org/10.3109/0284186X.2011.648338] [PMID: 22283470] ]
Currently available tests are insufficient to distinguish patients with EOC from normal individuals. Plasma specimens of EOC patients and normal individuals were analyzed using UPLC/QTOF/MS. Eight biomarkers were identified which may serve as novel biomarkers for diagnosis. Fig. (10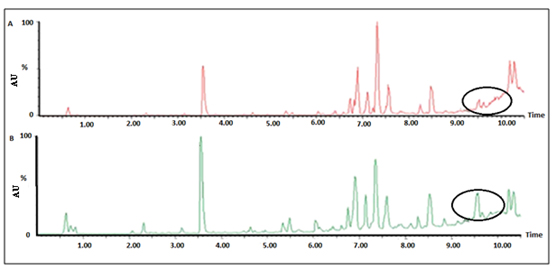 ) shows the application of UPLC in the diagnosis of EOC.
) shows the application of UPLC in the diagnosis of EOC.
ADME [66Yan, F.; Sun, M.; Hang, T.; Sun, J.; Zhou, X.; Deng, X.; Ge, L.; Qian, H.; Ya, D.; Huang, W. A rapid and sensitive UPLC-MS/MS method for determination of HZ08 in rat plasma and tissues: application to a pharmacokinetic study of liposome injections. J. Pharm. Biomed. Anal., 2015, 102, 246-252.
[http://dx.doi.org/10.1016/j.jpba.2014.09.017] [PMID: 25305722] , 67Karoly, T.; Monika, V. Solubility, Delivery and ADME Problems of Drugs and Drug-Candidates. Pharmacology and Drug Saftey; Bentham: Budapest, 2011, pp. 3-32.]
ADME studies include absorption, digestion, metabolism, and elimination. Yan developed and validated the sensitive UPLC-MS/MS method for the pharmacokinetic studies of HZ08 liposome injection in plasma and tissues of rats to study plasma kinetic and tissue distribution respectively.
Drug Abuse [68Hegstad, S.; Hermansson, S.; Betnér, I.; Spigset, O.; Falch, B.M. Screening and quantitative determination of drugs of abuse in diluted urine by UPLC-MS/MS. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci., 2014, 947-948, 83-95.
[http://dx.doi.org/10.1016/j.jchromb.2013.12.014] [PMID: 24413020] ]
UPLC-MS/MS method can be used to develop and evaluate a fast, robust and specific screening platform for the determination and quantification of a variety of commonly used drugs of abuse (opioids, benzodiazepines etc) in urine.
 |
Fig. (9) UPLCTM chromatograms of FAA profiles for the wine set fermented with the yeast Fermicru 4F9, (a) control wine, (b) 3 months, and (c) 6 months on-lees maturation. |
 |
Fig. (10) Plots from UPLC/QTOF/MS. A and B shows the typical total ion current chromatograms of plasma obtained from an EOC patient and a normal control, respectively. |
CONCLUSION
UPLC is one of the most important tools in analytical chemistry which increases the speed, resolution, and sensitivity of the chromatographic analysis and decreases the time, solvent consumption and cost involved. The peaks obtained through UPLC have decreased noise and better signal to noise ratio. It gives sharp and narrow peaks of more or less all categories of pharmaceutical drugs. It also facilitates the analysis of complex mixtures in less time and the peaks obtained through this method depicts more information which is more clearer in comparison to the peak obtained through HPLC. This method is widely used for the analysis of different pharmaceuticals such as amino acids, peptide mapping, glycans analysis, phenotyping, drug discovery, metabolomics etc. This technology thus creates a new opportunity for business profitability in highly efficient manner and allows the product to be introduced to the market in less time.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
Decleared None.
REFERENCES
| [1] | Quanyun, A.X. Ultra-High Performance Liquid Chromatography and Its Applications; John Wiley & Sons: New Jersey, 2013. |
| [2] | Chesnut, S.M.; Salisbury, J.J. The role of UHPLC in pharmaceutical development. J. Sep. Sci., 2007, 30(8), 1183-1190. [http://dx.doi.org/10.1002/jssc.200600505] [PMID: 17595953] |
| [3] | Fallas, M.M.; Neue, U.D.; Hadley, M.R.; McCalley, D.V. Further investigations of the effect of pressure on retention in ultra-high-pressure liquid chromatography. J. Chromatogr. A, 2010, 1217(3), 276-284. [http://dx.doi.org/10.1016/j.chroma.2009.11.041] [PMID: 20015498] |
| [4] | Nguyen, D.T.; Guillarme, D.; Heinisch, S.; Barrioulet, M.P.; Rocca, J.L.; Rudaz, S.; Veuthey, J.L. High throughput liquid chromatography with sub-2 microm particles at high pressure and high temperature. J. Chromatogr. A, 2007, 1167(1), 76-84. [http://dx.doi.org/10.1016/j.chroma.2007.08.032] [PMID: 17765255] |
| [5] | LCGC: Solution for Seperation Scientist. Available from: http://www.chromatographyonline.com |
| [6] | Nguyen, D.T.; Guillarme, D.; Rudaz, S.; Veuthey, J.L. Fast analysis in liquid chromatography using small particle size and high pressure. J. Sep. Sci., 2006, 29(12), 1836-1848. [http://dx.doi.org/10.1002/jssc.200600189] [PMID: 16970187] |
| [7] | Swartz, M.E. Ultra performance liquid chromatography (UPLC): An introduction, separation science re-defined. LCGC Suppl, 2005, 8, 8-14. |
| [8] | Jerkovich, A.D.; Mellors, J.S.; Jorgenson, J.W. Uplc: An Sensitive and High Throughput Analysis Over HPLC. LCGC, 2003, 21(7), 600-610. |
| [9] | MacNair, J.E.; Lewis, K.C.; Jorgenson, J.W. Ultrahigh-pressure reversed-phase liquid chromatography in packed capillary columns. Anal. Chem., 1997, 69(6), 983-989. [http://dx.doi.org/10.1021/ac961094r] [PMID: 9075400] |
| [10] | Beattie, K.; Joncour, J.S.; Lawson, , K. Ultra performance liquid chromatography coupled to orthogonal quadrupole TOF-MS (MS) for metabolite identification. LC GC North America, 2005, 22-30. |
| [11] | Wang, W.; Wang, S.; Tan, S.; Wen, M.; Qian, Y.; Zeng, X.; Guo, Y.; Yu, C. Detection of urine metabolites in polycystic ovary syndrome by UPLC triple-TOF-MS Clin. Chim.Acta, 2015, 448, 39-47. |
| [12] | Wu, N.; Lippert, J.A.; Lee, M.L. Practical aspects of ultrahigh pressure capillary liquid chromatography. J. Chromatogr. A., 2001, 911(1), 1-12. [http://dx.doi.org/10.1016/S0021-9673(00)01188-2] [PMID: 11269586] |
| [13] | Pidgeon, C. Advanced Tutorials for the Biomedical Sciences; Wiley-VCH: Canada, 1996, pp. 119-125. |
| [14] | Dong, M.W. Modern HPLC for Practicing Scientists; Wiley Interscience: New Jersey, 2006, pp. 35-37. [http://dx.doi.org/10.1002/0471973106] |
| [15] | Van Deemter, J.J.; Zuiderweg, E.J.; Klinkenberg, A. Longitudinal diffusion and resistance to mass transfer as causes of non ideality in chromatography. Chem. Eng. Sci., 1956, 5, 271-289. [http://dx.doi.org/10.1016/0009-2509(56)80003-1] |
| [16] | David, G.W. Pharmaceutical analysis. Sec edition; Elsevier Churchill Livingstone: UK, 2005, pp. 224-225. |
| [17] | Roge, A.B.; Firke, S.N.; Dhane, R.M.; Gunjkar, V.J.; Vadvalkar, S.M. Novel achievement of HPLC: UPLC. Int. J. Pharm. Tech. Res., 2011, 3(3), 1423-1429. |
| [18] | Yang, Y.; Hodges, C.C. Assay transfer from HPLC to UPLC for higher analysis throughput. LC GC Chromatography, 2005, 31-35. |
| [19] | Nováková, L.; Solichová, D.; Solich, P. Advantages of ultra performance liquid chromatography over high-performance liquid chromatography: comparison of different analytical approaches during analysis of diclofenac gel. J. Sep. Sci., 2006, 29(16), 2433-2443. [http://dx.doi.org/10.1002/jssc.200600147] [PMID: 17154123] |
| [20] | Wu, N.; Lippert, J.A.; Lee, M.L. Practical aspects of ultrahigh pressure capillary liquid chromatography. J. Chromatogr. A., 2001, 911(1), 1-12. [http://dx.doi.org/10.1016/S0021-9673(00)01188-2] [PMID: 11269586] |
| [21] | Sridhar, S.; Divya, S.; Sridhar, S.; Madhuri, R.; Sudhakar, M. UPLC-A dynamic and expeditious approach to liquid chromatography. Int. J. Pharm. Chem. Bio.Sci, 2013, 3(4), 1139-1152. |
| [22] | Gaikwad, P.V. Ultra Performance Liquid Chromatography: A recent novel development in HPLC. Inter. J. of Compre. Pharm., 2010, 2(08), 1-3. |
| [23] | Prathap, G.M.; Nishat, A. Ultra performance liquid chromatography: A chromatography technique. Int. J. Pharmacy, 2013, 3(1), 251-260. |
| [24] | Lars, Y.; Honore, H.S. On-line turbulent-flow chromatography-high-performance liquid chromatography-mass spectrometry for fast sample preparation and quantitation. J. Chromatogr, 2003, A 1020, 59-67. |
| [25] | McLoughlin, D.A.; Olah, T.V.; Gilbert, J.D. A direct technique for the simultaneous determination of 10 drug candidates in plasma by liquid chromatography-atmospheric pressure chemical ionization mass spectrometry interfaced to a Prospekt solid-phase extraction system. J. Pharmaceut. Biomed., 1997, 15, 1893-1901. |
| [26] | Broske, A.D. Agilent technologies application note, 2004. Available at: https://www.agilent.com/cs/library/primers/Public/5991-3326EN_SPHB.pdf |
| [27] | Acquity UPLC column. Available at: https://www.waters.com/webassets/cms/library/docs/720001140en.pdf, |
| [28] | Agilent Technologies. Available at: https://www.agilent.com/cs/library/catalogs/public/5991-1059EN%20LC_Columns.pdf, |
| [29] | Global Manufacturer pages. Available at: http://http://www.mfgpages.com/, |
| [30] | Phenomenex. Available at: http://http://www.phenomenex.com/, |
| [31] | Mezcua, M.; Agüera, A.; Lliberia, J.L.; Cortés, M.A.; Bagó, B.; Fernández-Alba, A.R. Application of ultra performance liquid chromatography-tandem mass spectrometry to the analysis of priority pesticides in groundwater. J. Chromatogr. A, 2006, 1109(2), 222-227. [http://dx.doi.org/10.1016/j.chroma.2006.01.024] [PMID: 16451801] |
| [32] | Lauber, M.A.; Koza, S.M.; McCall, S.A.; Alden, B.A.; Iraneta, P.C.; Fountain, K.J. High-resolution peptide mapping separations with MS-friendly mobile phases and charge-surface-modified C18. Anal. Chem., 2013, 85(14), 6936-6944. [http://dx.doi.org/10.1021/ac401481z] [PMID: 23772755] |
| [33] | Salane, K.; Stoffolano, P.J.; Robinson, E.; Eichhold, T. F; Hoke, I. S.; Baker, H.; Richardson, T. R.; Wehmeyer, E. C.; Kenneth, R. The evaluation and application of UPLC for the rapid analysis of dose formulations. LC GC, 2005, 23(Suppl.), 36. |
| [34] | Salane, K.; Peter, J.; Eric, R.; Thomas, E.; Steven, H.; Timothy, R.; Eloise, C.; Kenneth, R. The evaluation and application of UPLC for the rapid analysis of dose formulations. Sep. Sci. Red., 2005, 23(5), 36-39. |
| [35] | Yang, G.L.; Yang, L.W.; Li, Y.X.; Cao, H.; Zhou, W.L.; Fang, Z.J.; Zhou, H.B.; Mo, J.L.; Xiao, S.X.; Lin, H.R. Applications of ultra-performance liquid chromatography to traditional Chinese medicines. J. Chromatogr. Sci., 2010, 48(1), 18-21. [http://dx.doi.org/10.1093/chromsci/48.1.18] [PMID: 20056030] |
| [36] | Petrovic, M.; Gros, M.; Barcelo, D. Multi-residue analysis of pharmaceuticals in wastewater by ultra-performance liquid chromatography-quadrupole-time-of-flight mass spectrometry. J. Chromatogr. A, 2006, 1124(1-2), 68-81. [http://dx.doi.org/10.1016/j.chroma.2006.05.024] [PMID: 16759662] |
| [37] | Mao, J.; Xu, Y.; Lu, B.; Liu, J.; Hong, G.; Zhang, Q.; Sun, S.; Zhang, J. Simultaneous determination of nicotine and its nine metabolites in rat blood utilizing microdialysis coupled with UPLC-tandem mass spectrometry for pharmacokinetic application. Anal. Bioanal. Chem., 2015, 407(14), 4101-4109. [http://dx.doi.org/10.1007/s00216-015-8643-0] [PMID: 25824453] |
| [38] | Tang, Y.N.; Pang, Y.X.; He, X.C.; Zhang, Y.Z.; Zhang, J.Y.; Zhao, Z.Z.; Yi, T.; Chen, H.B. UPLC-QTOF-MS identification of metabolites in rat biosamples after oral administration of Dioscorea saponins: a comparative study. J. Ethnopharmacol., 2015, 165(165), 127-140. [http://dx.doi.org/10.1016/j.jep.2015.02.017] [PMID: 25698242] |
| [39] | Zeng, W.; Han, H.; Tao, Y.; Yang, L.; Wang, Z.; Chen, K. Identification of bio-active metabolites of gentiopicroside by UPLC/Q-TOF MS and NMR. Biomed. Chromatogr., 2013, 27(9), 1129-1136. [http://dx.doi.org/10.1002/bmc.2917] [PMID: 23733682] |
| [40] | Dhekale, N.H.; Bindu, K.H.; Kirankumar, K.Y.; Gore, A.H.; Anbhule, P.V.; Kolekar, G.B. Development and optimization of a multivariate RP-UPLC method for determination of telmisartan and its related substances by applying a two-level factorial design approach: Application to quality control study. Anal. Methods, 2014, 6(14), 5168-5182. [http://dx.doi.org/10.1039/c3ay42260g] |
| [41] | Srivastava, B.; Sharma, B.K.; Baghel, U.S.; Yashwant, S.N. Ultra performance liquid chromatography: A chromatography technique. Int. J. Pharm. Qua. Assur., 2010, 2(1), 19-25. |
| [42] | Lotfy, H.M.; Saleh, S.S.; Hassan, N.Y.; Salem, H. Development and validation of impurity-profiling UPLC method for the determination of sodium cromoglicate and tetryzoline hydrochloride: Application on rabbit aqueous humor. J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci., 2015, 1006(1006), 121-129. [http://dx.doi.org/10.1016/j.jchromb.2015.10.020] [PMID: 26547297] |
| [43] | Prakash, L.; Malipeddi, H.; Subbaiah, B.V.; Lakka, N.S. Impurity profiling and a stability-indicating UPLC method development and validation for the estimation of related impurities of halobetasol propionate in halobetasol propionate 0.05% (w/w) cream. J. Chromatogr. Sci., 2015, 53(1), 112-121. [http://dx.doi.org/10.1093/chromsci/bmu027] [PMID: 24795078] |
| [44] | Shaligram, S.R.; Ajameri, A.; Mody, R.; Padmaja, P. Development and validation of RP-HPLC and RP-UPLC methods for quantification of erythropoietin formulated with human serum albumin. J. Pharm. Anal., 2012, 2(2), 160-165. [http://dx.doi.org/10.1016/j.jpha.2011.11.006] |
| [45] | Ram, V.; Kher, G.; Dubal, K.; Dodiya, B.; Joshi, H. Development and validation of a stability indicating UPLC method for determination of ticlopidine hydrochloride in its tablet formulation. Saudi Pharm. J., 2011, 19(3), 159-164. [http://dx.doi.org/10.1016/j.jsps.2011.03.005] [PMID: 23960754] |
| [46] | Pradipbhai, D.K.; Sharma, M.; Garg, P.; Thota, J.R.; Ragampeta, S.; Talluri, M.V. Characterization of stress degradation products of mirabegron using UPLC-QTOF-MS/MS and in silico toxicity predictions of its degradation products. RSC Adv., 2015, 5, 31024-31038. [http://dx.doi.org/10.1039/C5RA01711D] |
| [47] | Karunakaran, K.; Navaneethan, G.; Elango, K.P. Development of a new RP-UPLC method for the determination of rabeprazole sodium in pharmaceutical formulation and application in dissolution studies. Trop. J. Pharm. Res., 2011, 10(5), 655. |
| [48] | Yadav, M.; Rao, R.; Kurani, H.; Singhal, P.; Goswami, S.; Shrivastav, P.S. Application of a rapid and selective method for the simultaneous determination of protease inhibitors, lopinavir and ritonavir in human plasma by UPLC-ESI-MS/MS for bioequivalence study in Indian subjects. J. Pharm. Biomed. Anal., 2009, 49(4), 1115-1122. [http://dx.doi.org/10.1016/j.jpba.2009.02.010] [PMID: 19282124] |
| [49] | Pidpruzhnykov, Y.V.; Sabko, V.E.; Iurchenko, V.V.; Zupanets, I.A. UPLC-MS/MS method for bioequivalence study of oral drugs of meldonium. Biomed. Chromatogr., 2012, 26(5), 599-605. [http://dx.doi.org/10.1002/bmc.1703] [PMID: 21915891] |
| [50] | Zhang, S.; Cheng, X.; Wang, Y.; Fan, J.; Li, R.; Zhou, S.; Liu, S.; Shi, J.; Sun, J.; Hu, Y.; Xu, C.; Wu, C.; Chang, X.; Tang, L.; Zhou, Z. Ninety day toxicity and toxicokinetics of fluorochloridone after oral administration in rats. Int. J. Environ. Res. Public Health, 2015, 12(5), 4942-4966. [http://dx.doi.org/10.3390/ijerph120504942] [PMID: 25955529] |
| [51] | Ding, G.; Zhang, X. A picture of polar iodinated disinfection byproducts in drinking water by (UPLC/)ESI-tqMS. Environ. Sci. Technol., 2009, 43(24), 9287-9293. [http://dx.doi.org/10.1021/es901821a] [PMID: 20000522] |
| [52] | Carlier, M. Quantification of seven β- lactam antibiotics and two β- lactamase inhibitors in human plasma using a validated UPLC-MS/MS method. Int. J. Antimicrob. Agents, 2012, 40(5), 416-422. |
| [53] | Thomas, T.; Petrie, K.; Shim, J.; Abildskov, K.M.; Westhoff, C.L.; Cremers, S. A UPLC-MS/MS method for therapeutic drug monitoring of etonogestrel. Ther. Drug Monit., 2013, 35(6), 844-848. [http://dx.doi.org/10.1097/FTD.0b013e31829a10fa] [PMID: 24081205] |
| [54] | Stuart, A. Analysis of explosives using Ultra Performance Liquid Chromatography with UV and/ or Mass Spectroscopy Detection. J. Ener. Mat., 2008, 26(4), 197-206. |
| [55] | Schummer, C.; Sassel, J.; Bonenberger, P.; Moris, G. Low-level detections of Sudan I, II, III and IV in spices and Chili-containing foodstuffs using UPLC-ESI-MS/MS. J. Agric. Food Chem., 2013, 61(9), 2284-2289. [http://dx.doi.org/10.1021/jf400602a] [PMID: 23390927] |
| [56] | Chevelle, A.C.; Stuart, A.O.; Thomas, A.F. Improved methodology for monitoring poly(amidoamine) dendrimers surface transformations and product quality by ultra performance liquid chromatography. J. Nanomat., 2008, 1-7. [http://dx.doi.org/10.1155/2008/456082] |
| [57] | Biricova, V.; Laznickova, A. Dendrimers: Analytical characterization and applications. Bioorg. Chem., 2009, 37(6), 185-192. [http://dx.doi.org/10.1016/j.bioorg.2009.07.006] [PMID: 19703699] |
| [58] | Quanyun, A.X. Ultra-High Performance Liquid Chromatography and Its Applications; Wiley & Sons: New Jersey, 2013, pp. 197-234. |
| [59] | Spácil, Z.; Nováková, L.; Solich, P. Analysis of phenolic compounds by high performance liquid chromatography and ultra performance liquid chromatography. Talanta, 2008, 76(1), 189-199. [http://dx.doi.org/10.1016/j.talanta.2008.02.021] [PMID: 18585262] |
| [60] | Pandey, R.; Chandra, P.; Srivastva, M.; Arya, K.R.; Shukla, P.K.; Kumar, B. A rapid analytical method for characterization and simultaneous quantitative determination of phytoconstituents in Piper betle landraces using UPLC-ESI-MS/MS. Anal. Methods, 2014, 6, 7349-7360. [http://dx.doi.org/10.1039/C4AY00975D] |
| [61] | Hasegawa, T.; Takahashi, K.; Saijo, M.; Ishii, T.; Nagata, T. Rapid determination of theophylline, theobromine and caffeine in dietary supplements containing guarana by ultra-performance liquid chromatography. Shokuhin Eiseigaku Zasshi, 2009, 50(6), 304-310. [http://dx.doi.org/10.3358/shokueishi.50.304] [PMID: 20065620] |
| [62] | Chen, X.J.; Ji, H.; Zhang, Q.W.; Tu, P.F.; Wang, Y.T.; Guo, B.L.; Li, S.P. A rapid method for simultaneous determination of 15 flavonoids in Epimedium using pressurized liquid extraction and ultra-performance liquid chromatography. J. Pharm. Biomed. Anal., 2008, 46(2), 226-235. [http://dx.doi.org/10.1016/j.jpba.2007.09.016] [PMID: 17961954] |
| [63] | Castro-Perez, J.M.; Roddy, T.P.; Shah, V.; McLaren, D.G.; Wang, S.P.; Jensen, K.; Vreeken, R.J.; Hankemeier, T.; Johns, D.G.; Previs, S.F.; Hubbard, B.K. Identifying static and kinetic lipid phenotypes by high resolution UPLC-MS: unraveling diet-induced changes in lipid homeostasis by coupling metabolomics and fluxomics. J. Proteome Res., 2011, 10(9), 4281-4290. [http://dx.doi.org/10.1021/pr200480g] [PMID: 21744776] |
| [64] | Fiechte, G.; Mayer, H.K. UPLC analysis of free amino acids in wines: Profiling of on-leesaged wines. J. Chromato. B., 2010, 879, 1361-1366. [http://dx.doi.org/10.1016/j.jchromb.2011.02.005] |
| [65] | Fan, L.; Zhang, W.; Yin, M.; Zhang, T.; Wu, X.; Zhang, H.; Sun, M.; Li, Z.; Hou, Y.; Zhou, X.; Lou, G.; Li, K. Identification of metabolic biomarkers to diagnose epithelial ovarian cancer using a UPLC/QTOF/MS platform. Acta Oncol., 2012, 51(4), 473-479. [http://dx.doi.org/10.3109/0284186X.2011.648338] [PMID: 22283470] |
| [66] | Yan, F.; Sun, M.; Hang, T.; Sun, J.; Zhou, X.; Deng, X.; Ge, L.; Qian, H.; Ya, D.; Huang, W. A rapid and sensitive UPLC-MS/MS method for determination of HZ08 in rat plasma and tissues: application to a pharmacokinetic study of liposome injections. J. Pharm. Biomed. Anal., 2015, 102, 246-252. [http://dx.doi.org/10.1016/j.jpba.2014.09.017] [PMID: 25305722] |
| [67] | Karoly, T.; Monika, V. Solubility, Delivery and ADME Problems of Drugs and Drug-Candidates. Pharmacology and Drug Saftey; Bentham: Budapest, 2011, pp. 3-32. |
| [68] | Hegstad, S.; Hermansson, S.; Betnér, I.; Spigset, O.; Falch, B.M. Screening and quantitative determination of drugs of abuse in diluted urine by UPLC-MS/MS. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci., 2014, 947-948, 83-95. [http://dx.doi.org/10.1016/j.jchromb.2013.12.014] [PMID: 24413020] |