- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

The Open Bioactive Compounds Journal
(Discontinued)
ISSN: 1874-8473 ― Volume 9, 2020
Biotransformation of Khellin to Khellol by Aspergillus Niger and the Evaluation of their Biological Activities
Ahmed Ashour1, 2, Saleh El-Sharkawy2, 3, Mohamed Amer2, Fatma Abdel Bar2, Amira Mera1, 2, Toshiro Nagata1, Ryuichiro Kondo1, Kuniyoshi Shimizu1, *
Abstract
Biotransformation of khellin using Aspergillus niger ATCC 10549 resulted in the production of khellol. The biological activities of the transformed product and khellin were established by antioxidant and acetylcholine esterase inhibitory assays. Khellol exhibited a higher degree of antioxidant and acetylcholine esterase inhibitory activities compared to khellin. This is the first report on the biotransformation of khellin by microorganisms and the first evaluation of the neuroprotective activity of either khellin or khellol.
Article Information
Identifiers and Pagination:
Year: 2013Volume: 4
First Page: 1
Last Page: 3
Publisher Id: TOBCJ-4-1
DOI: 10.2174/1874847320130628001
Article History:
Received Date: 15/5/2013Revision Received Date: 23/5/2013
Acceptance Date: 4/6/2013
epreprint1/7/2013
Electronic publication date: 4/9/2013
Collection year: 2013
open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
* Address correspondence to this author at the Department of Agro-environmental Sciences, Faculty of Agriculture, Kyushu University, 6-10-1 Hakozaki, Higashi-ku, Fukuoka 812-8581 Japan; E-mail: shimizu@agr.kyushu-u.ac.jp
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 15-5-2013 |
Original Manuscript | Biotransformation of Khellin to Khellol by Aspergillus Niger and the Evaluation of their Biological Activities | |
1. INTRODUCTION
Microbial transformations have been widely exploited in the preparation of many useful chemical products [1Hufford C, Badria F, Abou-Karam M, Shier W, Rogers R. Preparation, Characterization, and Antiviral Activity of Microbial Metabolites of Stemodin J Nat Prod 1991; 54: 1543-52.
[http://dx.doi.org/10.1021/np50078a008] [PMID: 1667410] -3Beukers R, Marx A, Zuidweg M. Microbial conversion as a
tool in the preparation of drugs In: Ariens E J, Ed. Drug design, 3. New York: Academic Press, Inc 1972.]. Khellin is an active compound extracted from Ammi visnaga Lam (Fam. Apiaceae), which is natural source of several furochromones. Khellin has been reported to have some biological effects such as relaxation of smooth muscle [4Kory R, Townes A, Mabe R, Dorris E, Meneely G. Drug Evaluation in Angina Pectoris: Khellin, Heparin, Peritrate Am
Heart J 1955; 50(2): 308-21.
[http://dx.doi.org/10.1016/0002-8703(55)90326-4] ] and prevention of stone formation associated with hyperoxaluria [5Vanachayangkul P, Byer K, Khan S, Butterweck V. An aqueous extract of Ammi visnaga fruits and its constituents khellin and visnagin prevent cell damage caused by oxalate in renal epithelial cells Phytomedicine 2010; 17(8-9): 653-8.
[http://dx.doi.org/10.1016/j.phymed.2009.10.011] [PMID: 2003611] [PMCID: PMC3618668] ], but no evidence concerning antioxidant or neuroprotective effects (acetylcholine esterase inhibitory activity) has been reported. This report describes the microbial transformation of khellin to khellol, and the antioxidant and neuroprotective evaluations of the metabolite.
2. MATERIALS AND METHODS
Plant Material
Khellin was isolated and purified from unused parts of Ammi visinaga grown in Egypt. Its identity and chemical structure was confirmed by comparative study to those cited in the literature [6Günaydin K, Beyazit N. The chemical investigation on the ripe fruits of Ammi visinaga (Lam.) Lamarck
growing in turkey Nat
Prod Res 2004; 18 (2): 169-75.
[http://dx.doi.org/10.1080/14786410310001608091] [PMID: 14984092] ].
General Experimental Procedures
Melting points were determined on a Fisher-Johns Scientific Co. melting point apparatus, USA. The 1H-, 13C-, APT, DEPT NMR spectra were analyzed on JEOL JNM ECA at 400 MHz for 1H- and 100 MHz for 13C-NMR spectra. TLC was performed on aluminum sheets precoated with 0.2-mm silica gel 60 F254 (Merck). Plates were developed in a solvent mixture of n-hexane-ethyl acetate (3:7, v/v), and the developed chromatograms were visualized under 254- and 365-nm UV light and the spots were made visible by spraying with vanillin/H2SO4 reagent before warming in an oven preheated to 110 °C for 5 min to develop yellow color for khellin and khellol.
Microorganisms
The microorganisms used in this work were provided by the culture collection of Mansoura University, Faculty of Pharmacy and were maintained on Potato-dextrose agar. The following microorganisms were screened for their ability to transform khellin: Aspergillus alliaceous UI 315, Aspergillus flavipes ATCC 11013, Aspergillus niger ATCC 10549, Aspergillus ochraceous NRRL 405, Bacillus cereus NRRLB-14591, Congronella butleri ATCC 22822, Cunninghamella blakesleeana MR 198, Cunninghamella echinulata NRRL 1382, Cunninghamella elegans NRRL 1392, Penicillium chrysogenum ATCC 10002, Rhodotorula rubra NRRL 1592, Streptomyces flocculus ATCC 25453, Streptomyces rutgersensis BT 256, Penicillium chrysogenum ATCC 9480, Rhodotorula rubra NRRL 1592, Absidia glauca ATCC 22752, Acrothecium capsici ATCC 10714, Debaromyces polymorphus ATCC 20280, Mucor mucedo UI 315, Penicillium vermiculatum NRRL 10009 and Saccharomyces cerevisiae ATCC 2366.
Fermentation Screening Procedure
Using a two-stage fermentation protocol [7El-Sharkawy S. Microbial conversion of tamoxifen Appl Microbiol
Biotechnol 1991; 35: 436-9.
[http://dx.doi.org/10.1007/BF00169745] ], screening was carried out by incubating the cultures while shaking at 200 rpm on New Brunswick Scientific G25 Gyratory shakers at 25 °C in a medium consisted of 2% glucose, 0.5% peptone, 0.5% yeast extract, 0.5% NaCl and 0.5% K2HPO4, adjusted to pH 7.0 before autoclaving for 15 min at 121 °C. Stage I culture was started by suspending spores and mycelia from filamentous fungal culture slants in 2 mL of sterile medium and transferring the suspension to 25 mL medium contained in 150-mL flasks. After incubation for 72 hours, stage II cultures were initiated by transferring 2 mL of stage I cultures to 150-mL flasks containing 25 mL medium. After 24 hours, khellin dissolved in dimethyl formamide (0.2 mg/mL medium) was added to the flasks. Culture controls consisted of fermentation blanks in which the organisms were grown under identical conditions but without substrate addition. Substrate controls were composed of a sterile medium to which the substrate was added and incubated without microorganisms. Samples (0.5 mL) of stage II cultures were extracted with EtOAc (2 mL × 3) every 24 hr for 15 days. The combined extracts were dried over anhydrous Na2SO4 and evaporated in vacuum at 40 °C. The dried extracts were reconstituted in 0.5 mL MeOH, applied to silica gel plates. The results of TLC analysis of EtOAc extracts of the culture broths of all microbes showed that Aspergillus niger ATCC 10549 could metabolize khellin to more polar metabolites after fourteen days. The fermentation screening procedures were repeated using Aspergillus niger ATCC 10549 to prove the reproducibility of the metabolite formation.
Scaled-up Reaction by Aspergillus Niger ATCC 10549
Khellin (500 mg) was evenly distributed among 25 (150-mL flasks), each containing 50 mL medium of 24-hr A. niger stage II cultures. The cultures were incubated for fourteen days (200 rpm, 25 °C) and extracted with EtOAc (1L × 3). The combined extracts were dried and evaporated to yield 430 mg yellow viscous residue. The residue was mixed with 400 mg of silica gel and placed on top of a silica gel column (l00 g, 1.5 × 72 cm). The column was isocratically eluted with 40% ethyl acetate in hexane. Fractions with identical Rf (TLC) were pooled.
Biological Evaluation
1. Acetylcholine Esterase Inhibitory Assay
AChE activity was measured using a microplate reader [8Ellman G, Lourtney D, Andres V, Gmelin G. A new and rapid colorimetric determination of
acetylcholinesterase activity Biochem
Pharmacol 1961; 7: 88-95.
[http://dx.doi.org/10.1016/0006-2952(61)90145-9] -9Mukherjee P, Kumar V, Mal M, Houghton P. In vitro acetylcholine esterase inhibitory activity of essential oil and its main constituents of Acorus calamus Planta Med 2007; 73: 283-5.
[http://dx.doi.org/10.1055/s-2007-967114] [PMID: 17286241] ]. Acetylcholine esterase enzyme hydrolyzes the substrate acetylthiocholine, resulting in the product thiocholine, which reacts with Ellman’s reagent (5,5-dithiobis [2-nitrobenzoic acid; (DTNB) to produce 2-nitrobenzoate-5-mercaptothiocholine and 5-thio-2-nitrobenzoate, which can be detected at 405 nm. One hundred microliters of Tris buffer (50 mM, pH= 8.0, 0.1% BSA, to stabilize the enzyme), 10 μl of 0.25 U/mL AChE enzyme, 10 μl of 10 mM DTNB, and 5 μl of compound per well at their maximum solubility [final concentrations of 0.73 mM (192.3 µg/mL) for khellin and 0.78 mM (192.3 µg/mL) for khellol] were added to 96-well plates, which were then incubated at 30 °C for 5 min; then, 5 μl of 75 mM acetylthiocholine was added to each well and the plate was incubated for an additional 10 min at 30 °C, after which the absorbance was measured at 405 nm. Galanthamine, 0.1 mM (38.46 µg/mL), was used as a positive control.
The percentage of inhibition was calculated using the following formula:
where ABlank is the absorbance of the blank in which the sample is replaced by buffer, Ablank 100% is the absorbance of the blank in which the sample and enzyme are replaced by buffer, A Sample is the absorbance of the sample, and A SampleBlank is the absorbance of the sample wells in which the enzyme is replaced by buffer.2. ORAC Assay
The oxygen radical anti-oxidant capacity (ORAC) assay measures the free radical scavenging activity of the sample and its ability to prevent the oxidative degeneration of the fluorescence of fluorescein after being mixed with the peroxyl radical generator 2,2`-azobis (2-amidino-propan) dihydrochloride (AAPH) at 37 °C. The ORAC method was used as previously described, with 96-well plates [10Prior R, Hoang H, Gu L, et al. Assays for hydrophilic and lipophilic antioxidant capacity (oxygen radical absorbance capacity (ORACFL) of plasma and other biological and food samples J Agric Food Chem 2003; 51: 3273-9.]. Briefly, 200 µl of 94.4-nM fluorescein was added to each 20-µl sample, Trolox, and phosphate buffer to reach 75 mM, pH 7.4 (as blank), and the mixture was incubated at 37 °C for 10 min. Seventy-five microliters of 31.7 mM AAPH was added to each well, and fluorescence degradation was measured for 90 min at 30-sec intervals. Excitation at 485 nm and emission at 525 nm were measured using a FlexStation 3 Microplate Reader (Molecular Devices, LLC, Sunnyvale, CA, USA), and data were managed by SoftMax Pro® 5.4.1 software. The standard curve was linear between 0 and 50 mM Trolox.
3. RESULTS AND DISCUSSION
Microbial transformation of khellin by A. niger produced one major spot on TLC with an Rf value of 0.2, which upon its isolation afforded a 150-mg (30%) colorless needle of the metabolite. This compound was identified by 1H- and 13C-NMR as well as co-chromatography with an authentic sample to be identified as khellol (Fig. 1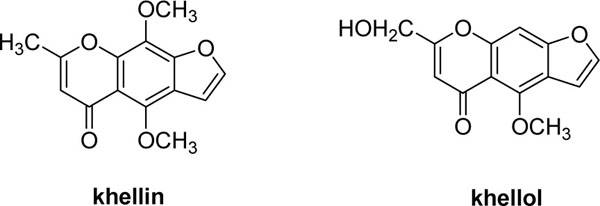 ). The data was further confirmed by consulting the literature [11Abou-Mustafa A, Saleh M, Elgamal A, Shalaby M, Duddeck H. A further contribution to the γ-pyrone constituents of Ammi visnaga fruits Planta Med 1990; 56(1): 134.
). The data was further confirmed by consulting the literature [11Abou-Mustafa A, Saleh M, Elgamal A, Shalaby M, Duddeck H. A further contribution to the γ-pyrone constituents of Ammi visnaga fruits Planta Med 1990; 56(1): 134.
[http://dx.doi.org/10.1055/s-2006-960912] [PMID: 17221392] ]. Ellman’s method was used to evaluate the neuroprotective activity through the inhibition of ACE by khellin and its metabolite at their higher concentration, 0.73 mM (192.3 µg/mL) for khellin and 0.78 mM (192.3 µg/mL) for khellol. Khellin showed weak inhibitory activity, 26.3%, at a higher concentration, compared with the positive control, galanthamine (0.1 mM, 38.46 µg/mL), which showed 93.4% ACEI, while higher inhibition was observed for khellol, which was 46.5% at the highest concentration (Fig. 2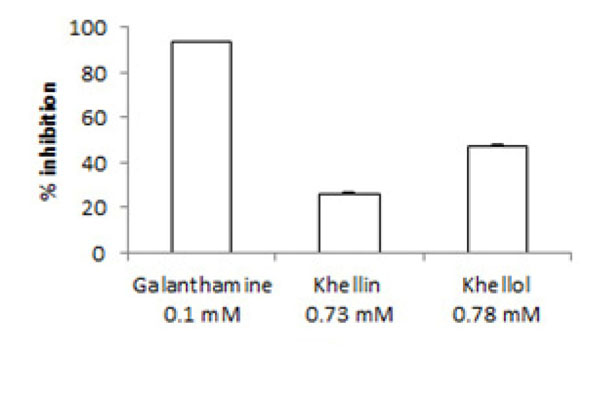 ).
).
 |
Fig. (1) Khellin and its metabolite khellol Biotransformation of Khellin to Khellol by Aspergillus Niger |
The results of ORAC assay (Table 1) showed khellol have higher antioxidant activity in comparison with khellin so it was concluded that the biotransformation of khellin greatly improved the neuroprotective and antioxidant activity through the formation of khellol as a metabolite. We also isolated khellol from the unused part of A. visinaga, proving that the plant metabolism mimics the microbial metabolism.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflicts of interest.
ACKNOWLEDGEMENT
The Egyptian Government is acknowledged for the fellowship support to Ahmed Adel Ashour.
REFERENCES
| [1] | Hufford C, Badria F, Abou-Karam M, Shier W, Rogers R. Preparation, Characterization, and Antiviral Activity of Microbial Metabolites of Stemodin J Nat Prod 1991; 54: 1543-52. [http://dx.doi.org/10.1021/np50078a008] [PMID: 1667410] |
| [2] | Badria F, Hufford C. Microbial transformations of stemodin, a Stemodia diterpene Phytochemistry 1991; 30(7): 2265-8. [http://dx.doi.org/10.1016/0031-9422(91)83626-V] |
| [3] | Beukers R, Marx A, Zuidweg M. Microbial conversion as a tool in the preparation of drugs In: Ariens E J, Ed. Drug design, 3. New York: Academic Press, Inc 1972. |
| [4] | Kory R, Townes A, Mabe R, Dorris E, Meneely G. Drug Evaluation in Angina Pectoris: Khellin, Heparin, Peritrate Am
Heart J 1955; 50(2): 308-21. [http://dx.doi.org/10.1016/0002-8703(55)90326-4] |
| [5] | Vanachayangkul P, Byer K, Khan S, Butterweck V. An aqueous extract of Ammi visnaga fruits and its constituents khellin and visnagin prevent cell damage caused by oxalate in renal epithelial cells Phytomedicine 2010; 17(8-9): 653-8. [http://dx.doi.org/10.1016/j.phymed.2009.10.011] [PMID: 2003611] [PMCID: PMC3618668] |
| [6] | Günaydin K, Beyazit N. The chemical investigation on the ripe fruits of Ammi visinaga (Lam.) Lamarck
growing in turkey Nat
Prod Res 2004; 18 (2): 169-75. [http://dx.doi.org/10.1080/14786410310001608091] [PMID: 14984092] |
| [7] | El-Sharkawy S. Microbial conversion of tamoxifen Appl Microbiol
Biotechnol 1991; 35: 436-9. [http://dx.doi.org/10.1007/BF00169745] |
| [8] | Ellman G, Lourtney D, Andres V, Gmelin G. A new and rapid colorimetric determination of
acetylcholinesterase activity Biochem
Pharmacol 1961; 7: 88-95. [http://dx.doi.org/10.1016/0006-2952(61)90145-9] |
| [9] | Mukherjee P, Kumar V, Mal M, Houghton P. In vitro acetylcholine esterase inhibitory activity of essential oil and its main constituents of Acorus calamus Planta Med 2007; 73: 283-5. [http://dx.doi.org/10.1055/s-2007-967114] [PMID: 17286241] |
| [10] | Prior R, Hoang H, Gu L, et al. Assays for hydrophilic and lipophilic antioxidant capacity (oxygen radical absorbance capacity (ORACFL) of plasma and other biological and food samples J Agric Food Chem 2003; 51: 3273-9. |
| [11] | Abou-Mustafa A, Saleh M, Elgamal A, Shalaby M, Duddeck H. A further contribution to the γ-pyrone constituents of Ammi visnaga fruits Planta Med 1990; 56(1): 134. [http://dx.doi.org/10.1055/s-2006-960912] [PMID: 17221392] |




