- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

Open Biological Sciences Journal
(Discontinued)
ISSN: 2352-6335 ― Volume 4, 2018
In Vitro Antimicrobial Activity and Chemical Composition of Two Essential Oils and Eugenol from Flower Buds of Eugenia caryophyllata
Natalija Atanasova-Pancevska1, *, Jane Bogdanov2, Dzoko Kungulovski1
Abstract
Background:
Aromatic plants and their essential oils have been used as food complements and medicine since the ancient times. Most of the researches today are pointed towards finding natural plant components which will have an antimicrobial action without having contraindications in human organism. Cloves, the dried aromatic flower buds of Eugenia caryophyllata, are widely used and known for their antimicrobial components.
Objectives:
The antimicrobial action of clove essential oils was tested against several microorganisms.
Methods:
Freshly isolated (hydrodistilled and dried) essential oil, and commercial essential oil were used and compared to pure eugenol, the most active and most important component. The microorganisms used in the research are Gram positive bacteria (Bacilus pumilus NCTC 8241, Bacillus subtilis ATCC 6633, Staphylococcus aureus ATCC 6538 and Sarcina lutea ATCC 9341), Gram negative bacteria (Escherichia coli ATCC 8739, Pseudomonas aeruginosa ATCC 9027), yeasts (Saccharomyces cerevisiae ATCC 9763, Candida albicans ATCC 10231) and molds (Penicillium sp., Aspergillus niger ATCC 16404, Aspergillus sojae). The microdilution test enabled determination of the minimal inhibitory concentration of three samples used in the experiment against all of test microorganism.
Results:
It was found that the eugenol had the strongest effect against microorganisms, with the exception of E. coli, where the freshly isolated essential oil had the strongest bactericide effect. The commercial essential oil had the weakest action against all of test microorganisms. The chemical composition of all three samples was determined via GC-MS and preliminary connection between the methods of preparation and storage of essential oils and their antimicrobial properties was proposed.
Conclusion:
Clove essential oil and eugenol possess broad spectrum of in vitro antibacterial and antifungal activity. So, they represent an alternative source of natural antimicrobial substances for use to prevent the growth of different bacteria, yeasts and molds.
Article Information
Identifiers and Pagination:
Year: 2017Volume: 3
First Page: 16
Last Page: 25
Publisher Id: BIOLSCI-3-16
DOI: 10.2174/2352633501703010016
Article History:
Received Date: 16/01/2017Revision Received Date: 03/03/2017
Acceptance Date: 03/03/2017
Electronic publication date: 28/04/2017
Collection year: 2017
open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
* Address correspondence to this author at the Department of Microbiology and Microbial Biotechnology, Institute of Biology, Faculty of Natural Sciences and Mathematics, University “Ss. Cyril and Methodius”, Arhimedova 3, 1000 Skopje, Macedonia; Tel: +389 2 3249 628; Fax: +389 2 3228 141; E-mails: natalijaap@gmail.com, atanasovan@yahoo.com
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 16-01-2017 |
Original Manuscript | In Vitro Antimicrobial Activity and Chemical Composition of Two Essential Oils and Eugenol from Flower Buds of Eugenia caryophyllata | |
INTRODUCTION
The aromatic herbs and plants, and the essential oils (EO) derived thereof besides their use as flavor and fragrance materials [1Hoffman DL. The herb user’s guide wellingborough. UK: Thorsons Publishing Group 1987., 2Lawless J. The illustrated encyclopedia of essential oils shaftesbury. UK: Element Books Ltd. 1995.] have been extensively used for medicinal purposes and as antimicrobial agents [3Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev 1999; 12(4): 564-82.
[PMID: 10515903] , 4Grayer RJ, Harborne JB. A survey of antifungal compounds from higher plants, 1982–1993. Phytochemistry 1994; 37(1): 19-42.
[http://dx.doi.org/10.1016/0031-9422(94)85005-4] ]. The essential oils are composed of volatile hydrophobic organic compounds that are biosynthesized in plants via the secondary metabolic pathways. The EOs are known for their biological activity [5Adorjan B, Buchbauer G. Biological properties of essential oils: an updated review. Flavour Fragrance J 2010; 25: 407-26.
[http://dx.doi.org/10.1002/ffj.2024] ] and antioxidant properties [6Amorati R, Foti MC, Valgimigli L. Antioxidant activity of essential oils. J Agric Food Chem 2013; 61(46): 10835-47.
[http://dx.doi.org/10.1021/jf403496k] [PMID: 24156356] ] and their renewability and availability make them appealing for plethora of applications.
The medical use of EO has regained popularity in the last decade, due to concerns about the potential health risks connected to the indiscriminate and extended use of man-made antimicrobial agents [7Bakkali F, Averbeck S, Averbeck D, Idaomar M. Biological effects of essential oilsa review. Food Chem Toxicol 2008; 46(2): 446-75.
[http://dx.doi.org/10.1016/j.fct.2007.09.106] [PMID: 17996351] ]. This trend is reinforced by the long-term use of EO as natural antimicrobials and the studies that claim fewer side-effects associated with their use. Additionally, the microbial diseases/infections are making a “comeback” as a result of appearance of resistant strains. So in order to alleviate the above-mentioned problems, it is more than a necessity to find substitutes for the existing synthetic antimicrobials. Most scientists have turned to extracts derived from natural products [8Newman DJ, Cragg GM. Natural products as sources of new drugs over the last 25 years. J Nat Prod 2007; 70(3): 461-77.
[http://dx.doi.org/10.1021/np068054v] [PMID: 17309302] ]. Researchers around the world are trying to unravel the secret of the EO and their components and the exact details of their antimicrobial activity [7Bakkali F, Averbeck S, Averbeck D, Idaomar M. Biological effects of essential oilsa review. Food Chem Toxicol 2008; 46(2): 446-75.
[http://dx.doi.org/10.1016/j.fct.2007.09.106] [PMID: 17996351] , 9Carson CF, Hammer KA, Riley TV. Melaleuca alternifolia (Tea Tree) oil: a review of antimicrobial and other medicinal properties. Clin Microbiol Rev 2006; 19(1): 50-62.
[http://dx.doi.org/10.1128/CMR.19.1.50-62.2006] [PMID: 16418522] -13Hammer KA, Carson CF, Riley TV. Antifungal effects of Melaleuca alternifolia (tea tree) oil and its components on Candida albicans, Candida glabrata and Saccharomyces cerevisiae. J Antimicrob Chemother 2004; 53(6): 1081-5.
[http://dx.doi.org/10.1093/jac/dkh243] [PMID: 15140856] ].
Usually, in traditional and/or “alternative” medicine the use of various EO has been empirical and it does not take into consideration the variability of EO’s chemical composition and properties. For adequate and safe use of EO for medicinal purposes, a switch from empirical to quantitative approach, that takes into consideration all pertinent factors, is more than necessary. The first step of action is the control/monitoring of the EO composition (usually a complex mixture of volatile secondary metabolites). The second step, when it comes to antimicrobial testing, one should use standardized protocols (CLSI, standard strains) and investigate the main component(s) in parallel with the EO and with standard antibiotics/ antimycotics. Also the minimal inhibitory concentration (MIC) for pure substances should be expressed in appropriate units, as to allow direct and unambiguous comparison of the antimicrobial effectiveness.
The essential oils from many plants are known to possess antibacterial and antifungal activity [7Bakkali F, Averbeck S, Averbeck D, Idaomar M. Biological effects of essential oilsa review. Food Chem Toxicol 2008; 46(2): 446-75.
[http://dx.doi.org/10.1016/j.fct.2007.09.106] [PMID: 17996351] , 14Burt S. Essential oils: their antibacterial properties and potential applications in foodsa review. Int J Food Microbiol 2004; 94(3): 223-53.
[http://dx.doi.org/10.1016/j.ijfoodmicro.2004.03.022] [PMID: 15246235] -16Kalemba D, Kunicka A. Antibacterial and antifungal properties of essential oils. Curr Med Chem 2003; 10(10): 813-29.
[http://dx.doi.org/10.2174/0929867033457719] [PMID: 12678685] ]. From many previously performed in vitro antimicrobial studies using EO, it can be concluded that antimicrobial activity in most cases is connected to the abundance of phenolic compounds (thymol, carvacrol, eugenol, isoeugenol). The fungal pathogens are especially susceptible to phenolic compounds, particularly to eugenol [17López P, Sánchez C, Batlle R, Nerín C. Solid- and vapor-phase antimicrobial activities of six essential oils: susceptibility of selected foodborne bacterial and fungal strains. J Agric Food Chem 2005; 53(17): 6939-46.
[http://dx.doi.org/10.1021/jf050709v] [PMID: 16104824] -21Pinto E, Vale-Silva L, Cavaleiro C, Salgueiro L. Antifungal activity of the clove essential oil from Syzygium aromaticum on Candida, Aspergillus and dermatophyte species. J Med Microbiol 2009; 58(Pt 11): 1454-62.
[http://dx.doi.org/10.1099/jmm.0.010538-0] [PMID: 19589904] ].
The chemical composition of thymol and carvacrol based EO (i.e. Thyme oil, Origano oil) can be rather complex and variable which will make a systematic study quite challenging. It has to be stressed one more time the need for the determination of chemical composition, especially because it is well known that these naturally occurring phenols can be toxic if present in high concentrations.
The plant that has been used as a rich source of eugenol is Eugenia caryophyllata Eugenia aromaticum or Syzygium aromaticum L. Merr. and Perry). The tree (family ) is widely cultivated in Madagascar, Tanzania, Sri Lanka, Indonesia, India, and the aromatic dried flower buds are used for the production of clove oil usually by steam or hydrodistillation. Taking into consideration the high clove oil yield (10-20%), availability, affordability and high eugenol content (>70%), it is a good candidate for systematic antimicrobial studies. Additionally, the clove oil has a relatively simple and “constant” chemical composition with three main components: eugenol, 1, (70-88%), eugenyl acetate, 2, (4-15%) and the sesquiterpene, β-caryophyllene 3, (4-21%) [22European Pharmacopoeia (VII). 7th ed. Strasbourg: Council of Europe 2011.]. Sometimes, the isomeric sesquiterpene, α-humulene, 4, is present in small quantities, with most commonly the oxidized sesquiterpenes - β-caryophyllene epoxide and α-humulene epoxides.
The aim of this study was to further assess the antibacterial and antifungal activity of commercial essential oil from Eugenia caryophyllata, laboratory prepared (hydrodistilled) essential oil from Eugenia caryophyllata, and pure eugenol against some representative bacteria, yeasts and molds according to standardized in vitro antimicrobial screening protocol. The goal was to explore and see if the difference in the chemical composition would have an effect on the antimicrobial activity.
MATERIALS AND METHODS
Chemicals
Eugenol (99%, Reagent plus), β-caryophyllene (98.5%), α-humulene (96%) meta-chloroperoxybenzoic acid (77%), and dichloromethane (99.5%) were obtained from Sigma-Aldrich. Dimethyl sulfoxide (DMSO, 99.8%), sodium hydroxide (98%), diethyl ether (99%) were obtained from Merck. All the chemicals were used without further purification.
Plant Material And Essential Oils
The commercial essential oil was obtained from a local pharmacy and was from the Beolab Spa & Wellness Company. The dried flower buds of Eugenia caryophyllata with declared origin from Madagascar were obtained from a local supplier. The plant material was pulverized and was subjected to hydrodistillation for 3 h in Clevenger-type apparatus (for denser than water EO). The essential oil was separated and dried over sodium sulfate for a minimum of 10 minutes. The overall yield of the EO was 14.2% (v/w) established on dry basis. Portion of the hydro distilled oil (2 g) was dissolved in ether (20 mL) and subjected to extraction with 5% (w/w) sodium hydroxide (3x15 mL). The combined NaOH layers were back-extracted with ether (3x10 ml). The combined aqueous layer was transferred to Erlenmayer flask and acidified with concentrated HCl to a pH value of 2. The acidified mixture is transferred to separate funnel and extracted with ether (3 x 15 mL). The combined organic layers were washed with water (3 x 15 mL) and brine (1 x15 mL). The ether layer was dried over sodium sulfate and decanted to a pre-weighed round bottom flask. Removal of solvents via rotary evaporation gave light yellow liquid (1.42 g), which was eugenol with analytical purity, GC-MS 99% pure. The purity was confirmed by comparison with authentic sample (eugenol, 99+% Reagent plus, Sigma-Aldrich). All the samples (isolated eugenol, the commercial EO and hydro distilled EO) were subjected to density, refractive index measurements, and IR and GC-MS analyses prior to the antimicrobial testing. Throughout the study they were stored at 4oC in dark glass air-tight bottles. Appropriate volumes of the samples were diluted in 100% DMSO, at a maximum concentration of 100 μLmL-1 (v/v) for each experiment. The FT-IR spectra were recorded on Varian 3100-IR Excalibur series spectrophotometer as liquid films between sodium chloride plates. The refractive index measurements were carried out on a digital Kruss A. Optronic (Germany) refractometer equipped with accurate internal thermostating unit.
Essential Oil Analysis
The chemical composition of clove bud essential oils was determined using GC and GC-MS. Samples (100 mg) were placed in 10 mL volumetric flask and were filled to the mark with dichloromethane. Gas chromatography (GC) was performed on Agilent 6890 GC system, equipped with flame-ionization detector (FID) and Agilent 5973 GC auto sampler. HP-5 column (30 m x 0.25 mm, 0.25 µm film thickness) was employed and nitrogen was used as a carrier gas at constant flow of 1.0 mLmin-1. Sample amount injected was 1µL, with applied split ratio of 1:50 at 250°C. The GC conditions were the following: the oven temperature started at 40°C and held for 5 min, then heated at 5°Cmin-1 to 140 °C, held for 5 min, then heated to 260°C at 10 °Cmin-1 and held at the final temp for 10 min. The temperature of the detector was at 260°C. The GC-MS analyses were carried out on a Varian 450 GC fitted with a VF-5MS fused silica capillary column (5% diphenyl- 95% dimethyl polysiloxane, 30 m x 0.25mm i.d., film thickness 0.25 μm) interfaced to a Varian 300 single quadrupole mass spectrometer, operated by Varian MS Workstation software. The GC temperature program was identical as in the GC-FID analysis. Helium (purity 99.999%) was used as a carrier gas with a constant flow rate of 1 mLmin-1. One microliter of the samples was injected using Varian CP 8410 autosampler, with a 50:1 split ratio and injector temperature of 250 °C. The temperatures of the transfer line and ion source were 240°C and 250°C, respectively. All mass spectra were acquired in electron impact (EI) mode at 70 eV in a full scan mode, with a range of 40-350 amu at a rate of 2 scans sec-1. Data acquisition and processing were carried out with Varian MS Workstation software, with capability of mass spectral matching. Identification of the components was based on comparison of the retention times with those of authentic samples and on computer matching against commercial (NIST 05) and home-made library mass spectra built up from pure substances and components of known oils. The relative amounts of individual components were calculated based on the GC-FID peak areas without response factor corrections. To correctly assign the GC peaks of the isomeric sesquiterpene epoxides (of β-caryophyllene and α-humulene) partial epoxidations with meta-chloroperoxybenzoic acid (mCPBA) were carried using literature procedures [23Bombarda I, Gaydou EM, Smadja J, Faure R. Synthesis of oxygenated compounds derived from caryophyllene. Bull Soc Chim Fr 1995; 132: 836-42., 24Damodaran NP, De S. Studies in sesquiterpenes-XXXVIII. Structure of humulene epoxide-I and humulene epoxide-II. Tetrahedron 1968; 24: 4123-32.
[http://dx.doi.org/10.1016/0040-4020(68)88175-X] ]. β-Caryophyllene was reacted with 0.75 equivalent of mCPBA, at 0 °C for 30 min, in dichloromethane following the procedure of [23Bombarda I, Gaydou EM, Smadja J, Faure R. Synthesis of oxygenated compounds derived from caryophyllene. Bull Soc Chim Fr 1995; 132: 836-42.]. After subsequent work-up, the obtained crude product was analyzed by GC-MS (ca. 3:1 β-caryophyllene epoxide: starting material). α-Humulene was reacted with 1 equivalent of mCPBA in chloroform according to [24Damodaran NP, De S. Studies in sesquiterpenes-XXXVIII. Structure of humulene epoxide-I and humulene epoxide-II. Tetrahedron 1968; 24: 4123-32.
[http://dx.doi.org/10.1016/0040-4020(68)88175-X] ], and the crude product was analyzed by GC-MS (94% humulene epoxide II, 3% humulene epoxide I and ca. 1% starting material).
Test Organisms
The antimicrobial activity of the extracts was assessed against six bacterial strains, Bacillus subtilis ATCC 6633, Escherichia coli ATCC 8739, Staphylococcus aureus ATCC 6538, Pseudomonas aeruginosa ATCC 9027, Sarcina lutea ATCC 9341, Bacillus pumilus NCTC 8241; two yeasts: Saccharomyces cerevisiae ATCC 9763 and Candida albicans ATCC 10231; and three molds: Penicillium sp., Aspergillus niger ATCC 16404 and Aspergillus sojae.
Resazurin Micro-Titre Assay
The 96-well micro-titre assay using resazurin as the indicator of cell growth [25Sarker SD, Nahar L, Kumarasamy Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 2007; 42(4): 321-4.
[http://dx.doi.org/10.1016/j.ymeth.2007.01.006] [PMID: 17560319] , 26Genest S, Kerr C, Shah A, et al. Comparative bioactivity studies on two Mimosa species. BLACPMA 2008; 7: 38-43.] was employed for the determination of the minimum inhibitory concentration (MIC) of the active extracts.
Standardization Of Inoculum
The test organisms were sub-cultured onto fresh plates of Mueller- Hinton agar (Oxoid, UK) for 24 h at 37°C for bacteria and Saboraud dextrose agar (Oxoid, UK) for 5 - 7 days at 25°C for fungi. Colonies from these plates were suspended in Mueller-Hinton broth and Saboraud broth (Oxoid, UK) to a turbidity matching 0.5 McFarland standard (108 colony forming units (cfu) ml-1).
Antimicrobial Assay
Into each well of the 96-well micro-titre plate, 50 μL of the medium, 50 μL of the extract, 5 μL of the resazurin and 5 μL of the suspension were dropped. Concentrations of extract used were 100-0.049 μLmL-1. Dilutions were made using DMSO as solvent. Each plate was incubating for 24 hours at 37°C for bacteria and 5 - 7 days at 25°C for fungi, as described by [27Pretorius JC, Magama S, Zietsman PC. Growth inhibition of plants pathogenic bacteria and fungi by extracts from selected South African plant species. S Afr J Bot 2003; 69(2): 188-92.
[http://dx.doi.org/10.1016/S0254-6299(15)30344-6] ]. A positive control (containing inoculum but no essential oil) and negative control (containing essential oil but no inoculum) were included on each microplate. A colour change from blue to pink was indicative of microbial growth. Solutions of benzylpenicillin, ampicillin and streptomycin (for bacteria) and nystatin (for yeasts and molds) were used for control wells.
RESULTS AND DISCUSSION
Chemical Composition of Essential Oils
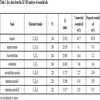
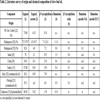
In our study, we have used commercial essential oil, hydro distilled one and pure isolated eugenol, 1. The clove buds (declared to be originating from Madagascar) were hydro distilled in Clevenger type of apparatus and the obtained EO was split into two parts. The first one was directly used in the antimicrobial studies, while the second part was used for the isolation of eugenol. The chemical composition of the isolated clove bud essential oil and the commercial clove bud essential oil was determined using GC-FID and GC-MS. Also, the purity of the isolated eugenol (99%) was determined by the GC-MS and refractive index measurement. From the obtained results it can be seen that in both the essential oils, the main component is eugenol 1 (Table 1). It is interesting to point out the absence of eugenyl acetate in the commercial essential oil and the presence of α-humulene. The chemical composition of the isolated essential oil is in agreement with the literature data (references within Table 2) [21Pinto E, Vale-Silva L, Cavaleiro C, Salgueiro L. Antifungal activity of the clove essential oil from Syzygium aromaticum on Candida, Aspergillus and dermatophyte species. J Med Microbiol 2009; 58(Pt 11): 1454-62.
[http://dx.doi.org/10.1099/jmm.0.010538-0] [PMID: 19589904] , 22European Pharmacopoeia (VII). 7th ed. Strasbourg: Council of Europe 2011., 28Gaydou EM, Randriamiharisoa R. Multidimensional analysis of gas chromatographic data, application to the differentiation of clove bud and clove stem essential oils from Madagascar. Perfum Flavor 1987; 12: 45-51.-33Chaieb K, Zmantar T, Ksouri R, et al. Antioxidant properties of the essential oil of Eugenia caryophyllata and its antifungal activity against a large number of clinical Candida species. Mycoses 2007; 50(5): 403-6.
[http://dx.doi.org/10.1111/j.1439-0507.2007.01391.x] [PMID: 17714361] ].
After the GC-FID/GC-MS analysis we have noted differences in the essential oils. It is interesting to note that both samples, based on the GC-MS analyses, contained, 2.3 and 2.7 wt.% of caryophyllene oxide, 5, which is an oxidation product of β-caryophyllene [34Turek C, Stintzing FC. Impact of different storage conditions on the quality of selected essential oils. Food Res Int 2012a; 46: 341-53.
[http://dx.doi.org/10.1016/j.foodres.2011.12.028] , 35Turek C, Stintzing FC. Stability of essential oils: A review. Compr Rev Food Sci Food Saf 2012b; 12: 40-53.
[http://dx.doi.org/10.1111/1541-4337.12006] ]. Additionally, the commercial essential oil contained significant amounts of β-caryophyllene, 3, (10.41%), and some of α-humulene, 4, (~2%), while the hydro distilled EO contained low amounts of β-caryophyllene, 3, (1.21%). With pure eugenol as a control we had a convenient control probe to test the essential oils with almost the same content of eugenol (84.8 and 86.3 wt.%), but different content of eugenyl acetate and β-caryophyllene. The chemical composition of the isolated essential oil is in agreement with the literature data (references within Table 2).
During our preparation we have thoroughly dried the hydro-distilled clove essential oil over sodium sulfate. We have postulated that the way commercial oils are manufactured they are not additionally dried. Over time and storage, the traces of water can hydrolyze the ester, eugenyl acetate, 2, and if it is originally present in small quantities it may not be detectable by GC analyses. In our case, we wanted to confirm the absence of 2, and to eliminate the possible decomposition and/or hydrolysis at the GC injector. For that purpose we have recorded the FT-IR spectra of all samples (commercial essential oil, the hydro distilled/dried essential oil and pure eugenol). The typical ester absorption band (1743 cm-1) was absent in the IR spectra of the commercial essential oil, but was present in hydrodistilled and dried essential oil.
Antimicrobial Activity
A few antimicrobial studies involving clove essential oils with chromatographically determined chemical composition have been done [33Chaieb K, Zmantar T, Ksouri R, et al. Antioxidant properties of the essential oil of Eugenia caryophyllata and its antifungal activity against a large number of clinical Candida species. Mycoses 2007; 50(5): 403-6.
[http://dx.doi.org/10.1111/j.1439-0507.2007.01391.x] [PMID: 17714361] , 21Pinto E, Vale-Silva L, Cavaleiro C, Salgueiro L. Antifungal activity of the clove essential oil from Syzygium aromaticum on Candida, Aspergillus and dermatophyte species. J Med Microbiol 2009; 58(Pt 11): 1454-62.
[http://dx.doi.org/10.1099/jmm.0.010538-0] [PMID: 19589904] , 19Chaieb K, Hajlaoui H, Zmantar T, et al. The chemical composition and biological activity of clove essential oil, Eugenia caryophyllata (Syzigium aromaticum L. Myrtaceae): a short review. Phytother Res 2007; 21(6): 501-6.
[http://dx.doi.org/10.1002/ptr.2124] [PMID: 17380552] , 36Leela NK, Sapna VP. Clove. In: Parthasarathy VA, Chempakam B, Zachariah TJ, Eds. Chemistry of Spices. 2008; pp. 146-64.
[http://dx.doi.org/10.1079/9781845934057.0146] ]. Chemical composition can vary, depending on the origin, storage of the clove buds, isolation and processing and storage [34Turek C, Stintzing FC. Impact of different storage conditions on the quality of selected essential oils. Food Res Int 2012a; 46: 341-53.
[http://dx.doi.org/10.1016/j.foodres.2011.12.028] -36Leela NK, Sapna VP. Clove. In: Parthasarathy VA, Chempakam B, Zachariah TJ, Eds. Chemistry of Spices. 2008; pp. 146-64.
[http://dx.doi.org/10.1079/9781845934057.0146] ], which in turn may affect the results of the in vitro antimicrobial studies.
The inhibitory activity of clove is due to the presence of several constituents, mainly eugenol, eugenyl acetate, beta-caryophyllene, 2-heptanone [33Chaieb K, Zmantar T, Ksouri R, et al. Antioxidant properties of the essential oil of Eugenia caryophyllata and its antifungal activity against a large number of clinical Candida species. Mycoses 2007; 50(5): 403-6.
[http://dx.doi.org/10.1111/j.1439-0507.2007.01391.x] [PMID: 17714361] ], and to a lesser extent because of their lower abundance- α-humulene, methyl salicylate, isoeugenol, methyl eugenol [37Giorgio P, Mario C, Paola M, Stefania Z, Antonella D, Bruno T. Chemical composition and antimicrobial activities of essential oil of Stachys glutinosa L. from Sardinia. Nat Prod Commun 2006; 1: 1133-6.].
Several studies have demonstrated potent antifungal [38Arina B, Iqbal A. In vitro fungitoxicity of the essential oil of Syzygium aromaticum. World J Microbiol Biotechnol 2012; 18(4): 317-9.-41Park MJ, Gwak KS, Yang I, et al. Antifungal activities of the essential oils in Syzygium aromaticum (L.) Merr. Et Perry and Leptospermum petersonii Bailey and their constituents against various dermatophytes. J Microbiol 2007; 45(5): 460-5.
[PMID: 17978807] ], antiviral [19Chaieb K, Hajlaoui H, Zmantar T, et al. The chemical composition and biological activity of clove essential oil, Eugenia caryophyllata (Syzigium aromaticum L. Myrtaceae): a short review. Phytother Res 2007; 21(6): 501-6.
[http://dx.doi.org/10.1002/ptr.2124] [PMID: 17380552] ] and antibacterial effects of clove [17López P, Sánchez C, Batlle R, Nerín C. Solid- and vapor-phase antimicrobial activities of six essential oils: susceptibility of selected foodborne bacterial and fungal strains. J Agric Food Chem 2005; 53(17): 6939-46.
[http://dx.doi.org/10.1021/jf050709v] [PMID: 16104824] , 42Cai L, Wu CD. Compounds from Syzygium aromaticum possessing growth inhibitory activity against oral pathogens. J Nat Prod 1996; 59(10): 987-90.
[http://dx.doi.org/10.1021/np960451q] [PMID: 8904847] -46Fu Y, Zu Y, Chen L, et al. Antimicrobial activity of clove and rosemary essential oils alone and in combination. Phytother Res 2007; 21(10): 989-94.
[http://dx.doi.org/10.1002/ptr.2179] [PMID: 17562569] ]. It has been proposed that the most active compounds contain the phenol functional group. These phenolic compounds sensitize the phospholipid bilayer of the microbial cytoplasmic membrane causing increased permeability, unavailability of vital intracellular constituents [47Juven BJ, Kanner J, Schved F, Weisslowicz H. Factors that interact with the antibacterial action of thyme essential oil and its active constituents. J Appl Bacteriol 1994; 76(6): 626-31.
[http://dx.doi.org/10.1111/j.1365-2672.1994.tb01661.x] [PMID: 8027009] ] and/or impairment of bacterial enzymes systems [48Farag RS. Antioxidant activity of some spice essential oils on linobic acid oxidation in aqueous media. JAOGS 1989; 66: 792-9.].
The 96-well micro-titre assay with resazurin [25Sarker SD, Nahar L, Kumarasamy Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 2007; 42(4): 321-4.
[http://dx.doi.org/10.1016/j.ymeth.2007.01.006] [PMID: 17560319] ] was employed to evaluate the antimicrobial activity of clove essential oil and eugenol. Blue-coloured suspensions are consistent with no growth of microorganisms, and red-coloured suspension are consistent with higher levels of microorganisms proliferation. This method is a nonradiometric, rapid, high-throughput assay that allows for the detection of bacterial activity with a high degree of confidence [49Yajko DM, Madej JJ, Lancaster MV, et al. Colorimetric method for determining MICs of antimicrobial agents for Mycobacterium tuberculosis. J Clin Microbiol 1995; 33(9): 2324-7.
[PMID: 7494021] -51Kumar M, Khan IA, Verma V, Kalyan N, Qazi GN. Rapid, inexpensive MIC determination of Mycobacterium tuberculosis isolates by using microplate nitrate reductase assay. Diagn Microbiol Infect Dis 2005; 53(2): 121-4.
[http://dx.doi.org/10.1016/j.diagmicrobio.2005.05.011] [PMID: 16168616] ]. The assay is based on the oxidation–reduction dye resazurin as a general indicator of cellular growth [52OBrien J, Wilson I, Orton T, Pognan F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur J Biochem 2000; 267(17): 5421-6.
[http://dx.doi.org/10.1046/j.1432-1327.2000.01606.x] [PMID: 10951200] ]. In the presence of metabolic activity, the dye is reduced and undergoes a colour change with fluorescence.
The results for antimicrobial properties of extracts are shown in Table 3. Eugenol, as a main component of extracts, demonstrated very strong activity against all tested microorganisms, with lowest MIC of 0.049 μLmL-1 for Bacillus subtilis ATCC 6633. With regard to antifungal activity, the clove extract was active against all the test fungal species (Table 3).The lowest MIC was for eugenol on Penicillium sp. (0.049 μLmL-1). The antimicrobial properties of clove essential oil were tested and showed inhibitory activity to Listeria monocytogenes, Campylobacter jejuni, Salmonella enteritidis, Bacillus cereus, Escherichia coli and Staphylococcus aureus [53Cressy HK, Jerrett AR, Osborne CM, Bremer PJ. A novel method for the reduction of numbers of Listeria monocytogenes cells by freezing in combination with an essential oil in bacteriological media. J Food Prot 2003; 66(3): 390-5.
[http://dx.doi.org/10.4315/0362-028X-66.3.390] [PMID: 12636290] , 54Velluti A, Sanchis V, Ramos AJ, Egido J, Marín S. Inhibitory effect of cinnamon, clove, lemongrass, oregano and palmarose essential oils on growth and fumonisin B1 production by Fusarium proliferatum in maize grain. Int J Food Microbiol 2003; 89(2-3): 145-54.
[http://dx.doi.org/10.1016/S0168-1605(03)00116-8] [PMID: 14623380] ].
Essential oils of clove possess high antimicrobial properties [16Kalemba D, Kunicka A. Antibacterial and antifungal properties of essential oils. Curr Med Chem 2003; 10(10): 813-29.
[http://dx.doi.org/10.2174/0929867033457719] [PMID: 12678685] ]. Clove oil was effective against E. coli, L monocytogenes, S. enteric [55Friedman M, Henika PR, Mandrell RE. Bactericidal activities of plant essential oils and some of their isolated constituents against Campylobacter jejuni, Escherichia coli, Listeria monocytogenes, and Salmonella enterica. J Food Prot 2002; 65(10): 1545-60.
[http://dx.doi.org/10.4315/0362-028X-65.10.1545] [PMID: 12380738] ]. The antibacterial activity of clove against two gram-negative bacteria, such as Pseudomonas fluorescens and Serratia liquefaciens, and four gram-positive bacteria, such as Brochothrix thermosphacta, Carnobacterium piscicola, Lactobacillus curvatus, and Lactobacillus sp., involved in meat spoilage, was found effective.
Generally, this study showed that clove oil was effective against both Gram-positive and Gram-negative bacteria, and yeasts and fungi, which is similar to other reports describing the use of essential oils. The present findings support, at least to some extent, the traditional uses of this plant, particularly its use for the treatment of bacterial and fungal infections and inflammation.
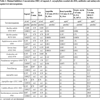
It was also found that the eugenol had the strongest effect against bacterial cultures, with the exception of E. coli, where the freshly isolated essential oil had the strongest bactericidal effect. Also, the freshly isolated essential oil had the strongest effect against the C. albicans, Important to note is that a similar study on antifungal activity of eugenol and clove essential oil on Candida and Aspergillus species was done by Pinto and co-workers [21Pinto E, Vale-Silva L, Cavaleiro C, Salgueiro L. Antifungal activity of the clove essential oil from Syzygium aromaticum on Candida, Aspergillus and dermatophyte species. J Med Microbiol 2009; 58(Pt 11): 1454-62.
[http://dx.doi.org/10.1099/jmm.0.010538-0] [PMID: 19589904] ]. Another peculiarity is that in their study they have used a commercial essential oil from Portugal, and based on their GC analysis they have not detected eugenyl acetate either. Taking into consideration that in our study the only major difference between the two EOs (freshly prepared and commercial) is the presence of eugenyl acetate, one is tempted to attribute the higher overall antimicrobial activity of the freshly hydrodistilled/dried EO to its presence. This assumption finds support in recent study by Chiaradia and co-workers [56Chiaradia V, Paroul N, Cansian RL, et al. Synthesis of eugenol esters by lipase-catalyzed reaction in solvent-free system. Appl Biochem Biotechnol 2012; 168(4): 742-51.
[http://dx.doi.org/10.1007/s12010-012-9814-5] [PMID: 22864649] ] who synthesized pure eugenyl acetate by lipase-catalyzed reaction in solvent-free system and tested its antimicrobial activity in parallel with eugenol employing diffusion disk technique. The eugenyl acetate led to an increase in the antimicrobial activity (16 bacteria tested) when compared to pure eugenol (i.e the acetylation of eugenol improved its antimicrobial properties). Further studies centered on the preparation and antimicrobial studies with surrogate EOs containing varying ratios of the three main components (eugenol, beta-caryophyllene and eugenyl acetate) are needed to establish the “true” contribution of each component. Also the minimal inhibitory concentration (MIC) for pure substances should be expressed in appropriate units, as to allow direct and unambiguous comparison of the antimicrobial effectiveness. The easiest way for comparison is to convert concentrations from μLmL-1 and μgmL-1 to μmol mL-1. The antimicrobial activity of eugenol can now be directly and unambiguously compared to the activity of the commercial antibiotics and antimycotics. From Table 3. it can be clearly seen, based on micromolar amounts, that eugenol is especially potent antifungal agent and is superior to man-made antimycotics. If one takes into consideration the high EO yield, availability, affordability and high eugenol content, the flower buds of Eugenia caryophyllata are more than obvious source of natural antimicrobials.
CONCLUSION
The results of our study confirmed that clove oil has a wide-spectrum antibacterial and antifungal activity. Even though it is quite difficult to attribute the biological activity of mixtures such as essential oils, it is not unreasonable to pinpoint the “major contributor”. In our particular case, it is eugenol, as confirmed by studies involving pure component and EOs containing high concentrations (84.8% and 86.29%) of eugenol. The outcome of these types of in vitro antimicrobial/antifungal tests depends on many factors, such as prior history of the plant material, the extraction method of EO, the storage conditions (Turek & Stintzing, 2012). All of the above-mentioned may affect the chemical composition of EO, and it is advisable to check the purity of EOs before in vitro antimicrobial tests. Despite the fact that the EOs are hydrophobic, the ones that contain larger quantities of phenolic compounds (ex. thymol,carvacrol, eugenol etc.) or contain hydrolysable compounds (ex. esters) should be dried after preparation and before testing. In order to avoid the ambiguities we propose, in addition to the determination of chemical composition by gas chromatographic techniques, to check the refractive index and the moisture content of the EOs.
In summary, this study confirmed that clove essential oil and eugenol possess broad spectrum of in vitro antibacterial and antifungal activity. Therefore it represents an alternative source of natural antimicrobial substances for use to prevent the growth of different bacteria, yeasts and molds. For standardization of the results of the antimicrobial studies involving essential oils, prior check of their chemical composition is highly advisable.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
Declared None.
REFERENCES
| [1] | Hoffman DL. The herb user’s guide wellingborough. UK: Thorsons Publishing Group 1987. |
| [2] | Lawless J. The illustrated encyclopedia of essential oils shaftesbury. UK: Element Books Ltd. 1995. |
| [3] | Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev 1999; 12(4): 564-82. [PMID: 10515903] |
| [4] | Grayer RJ, Harborne JB. A survey of antifungal compounds from higher plants, 1982–1993. Phytochemistry 1994; 37(1): 19-42. [http://dx.doi.org/10.1016/0031-9422(94)85005-4] |
| [5] | Adorjan B, Buchbauer G. Biological properties of essential oils: an updated review. Flavour Fragrance J 2010; 25: 407-26. [http://dx.doi.org/10.1002/ffj.2024] |
| [6] | Amorati R, Foti MC, Valgimigli L. Antioxidant activity of essential oils. J Agric Food Chem 2013; 61(46): 10835-47. [http://dx.doi.org/10.1021/jf403496k] [PMID: 24156356] |
| [7] | Bakkali F, Averbeck S, Averbeck D, Idaomar M. Biological effects of essential oilsa review. Food Chem Toxicol 2008; 46(2): 446-75. [http://dx.doi.org/10.1016/j.fct.2007.09.106] [PMID: 17996351] |
| [8] | Newman DJ, Cragg GM. Natural products as sources of new drugs over the last 25 years. J Nat Prod 2007; 70(3): 461-77. [http://dx.doi.org/10.1021/np068054v] [PMID: 17309302] |
| [9] | Carson CF, Hammer KA, Riley TV. Melaleuca alternifolia (Tea Tree) oil: a review of antimicrobial and other medicinal properties. Clin Microbiol Rev 2006; 19(1): 50-62. [http://dx.doi.org/10.1128/CMR.19.1.50-62.2006] [PMID: 16418522] |
| [10] | Cox SD, Mann CM, Markham JL, et al. The mode of antimicrobial action of the essential oil of Melaleuca alternifolia (tea tree oil). J Appl Microbiol 2000; 88(1): 170-5. [http://dx.doi.org/10.1046/j.1365-2672.2000.00943.x] [PMID: 10735256] |
| [11] | Cox SD, Mann CM, Markham JL, Gustafson JE, Warmington JR, Wyllie SG. Determining the antimicrobial actions of tea tree oil. Molecules 2001; 6(2): 87-91. [http://dx.doi.org/10.3390/60100087] |
| [12] | Di Pasqua R, Betts G, Hoskins N, Edwards M, Ercolini D, Mauriello G. Membrane toxicity of antimicrobial compounds from essential oils. J Agric Food Chem 2007; 55(12): 4863-70. [http://dx.doi.org/10.1021/jf0636465] [PMID: 17497876] |
| [13] | Hammer KA, Carson CF, Riley TV. Antifungal effects of Melaleuca alternifolia (tea tree) oil and its components on Candida albicans, Candida glabrata and Saccharomyces cerevisiae. J Antimicrob Chemother 2004; 53(6): 1081-5. [http://dx.doi.org/10.1093/jac/dkh243] [PMID: 15140856] |
| [14] | Burt S. Essential oils: their antibacterial properties and potential applications in foodsa review. Int J Food Microbiol 2004; 94(3): 223-53. [http://dx.doi.org/10.1016/j.ijfoodmicro.2004.03.022] [PMID: 15246235] |
| [15] | Dorman HJ, Deans SG. Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J Appl Microbiol 2000; 88(2): 308-16. [http://dx.doi.org/10.1046/j.1365-2672.2000.00969.x] [PMID: 10736000] |
| [16] | Kalemba D, Kunicka A. Antibacterial and antifungal properties of essential oils. Curr Med Chem 2003; 10(10): 813-29. [http://dx.doi.org/10.2174/0929867033457719] [PMID: 12678685] |
| [17] | López P, Sánchez C, Batlle R, Nerín C. Solid- and vapor-phase antimicrobial activities of six essential oils: susceptibility of selected foodborne bacterial and fungal strains. J Agric Food Chem 2005; 53(17): 6939-46. [http://dx.doi.org/10.1021/jf050709v] [PMID: 16104824] |
| [18] | Velluti A, Sanchis V, Ramos AJ, Marin S. Effect of essential oils of cinnamon, clove, lemon grass, oregano and palmarosa on growth of and fumonisin B1 production by Fusarium verticillioides in maize. J Sci Food Agric 2004; 84(10): 1141-6. [http://dx.doi.org/10.1002/jsfa.1769] |
| [19] | Chaieb K, Hajlaoui H, Zmantar T, et al. The chemical composition and biological activity of clove essential oil, Eugenia caryophyllata (Syzigium aromaticum L. Myrtaceae): a short review. Phytother Res 2007; 21(6): 501-6. [http://dx.doi.org/10.1002/ptr.2124] [PMID: 17380552] |
| [20] | Gayoso CW, Lima EO, Oliveira VT, et al. Sensitivity of fungi isolated from onychomycosis to Eugenia cariophyllata essential oil and eugenol. Fitoterapia 2005; 76(2): 247-9. [http://dx.doi.org/10.1016/j.fitote.2004.12.005] [PMID: 15752642] |
| [21] | Pinto E, Vale-Silva L, Cavaleiro C, Salgueiro L. Antifungal activity of the clove essential oil from Syzygium aromaticum on Candida, Aspergillus and dermatophyte species. J Med Microbiol 2009; 58(Pt 11): 1454-62. [http://dx.doi.org/10.1099/jmm.0.010538-0] [PMID: 19589904] |
| [22] | European Pharmacopoeia (VII). 7th ed. Strasbourg: Council of Europe 2011. |
| [23] | Bombarda I, Gaydou EM, Smadja J, Faure R. Synthesis of oxygenated compounds derived from caryophyllene. Bull Soc Chim Fr 1995; 132: 836-42. |
| [24] | Damodaran NP, De S. Studies in sesquiterpenes-XXXVIII. Structure of humulene epoxide-I and humulene epoxide-II. Tetrahedron 1968; 24: 4123-32. [http://dx.doi.org/10.1016/0040-4020(68)88175-X] |
| [25] | Sarker SD, Nahar L, Kumarasamy Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 2007; 42(4): 321-4. [http://dx.doi.org/10.1016/j.ymeth.2007.01.006] [PMID: 17560319] |
| [26] | Genest S, Kerr C, Shah A, et al. Comparative bioactivity studies on two Mimosa species. BLACPMA 2008; 7: 38-43. |
| [27] | Pretorius JC, Magama S, Zietsman PC. Growth inhibition of plants pathogenic bacteria and fungi by extracts from selected South African plant species. S Afr J Bot 2003; 69(2): 188-92. [http://dx.doi.org/10.1016/S0254-6299(15)30344-6] |
| [28] | Gaydou EM, Randriamiharisoa R. Multidimensional analysis of gas chromatographic data, application to the differentiation of clove bud and clove stem essential oils from Madagascar. Perfum Flavor 1987; 12: 45-51. |
| [29] | Srivastava AK, Srivastava SK, Syamsundar KV. Bud and leaf essential oil composition of Syzygium aromaticum from India and Madagascar. Flavour Fragrance J 2005; 20(1): 51-3. [http://dx.doi.org/10.1002/ffj.1364] |
| [30] | Pino JA, Marbot R, Aguero J, Fuentes V. Essential oil from buds and leaves of clove (Syzygium aromaticum (L.) Merr. et Perry) grown in Cuba. J Essent Oil Res 2001; 13(4): 278-9. [http://dx.doi.org/10.1080/10412905.2001.9699693] |
| [31] | Alma MH, Ertas M, Nitz S, Kollmannsberger H. Chemical composition and content of essential oil from the bud of cultivated turkish clove (Syzygium aromaticum L.). BioResources 2007; 2: 265-9. |
| [32] | Tomaino A, Cimino F, Zimbalatti V, et al. Influence of heating on antioxidant activity and the chemical composition of some spice essential oils. Food Chem 2005; 89(4): 549-54. [http://dx.doi.org/10.1016/j.foodchem.2004.03.011] |
| [33] | Chaieb K, Zmantar T, Ksouri R, et al. Antioxidant properties of the essential oil of Eugenia caryophyllata and its antifungal activity against a large number of clinical Candida species. Mycoses 2007; 50(5): 403-6. [http://dx.doi.org/10.1111/j.1439-0507.2007.01391.x] [PMID: 17714361] |
| [34] | Turek C, Stintzing FC. Impact of different storage conditions on the quality of selected essential oils. Food Res Int 2012a; 46: 341-53. [http://dx.doi.org/10.1016/j.foodres.2011.12.028] |
| [35] | Turek C, Stintzing FC. Stability of essential oils: A review. Compr Rev Food Sci Food Saf 2012b; 12: 40-53. [http://dx.doi.org/10.1111/1541-4337.12006] |
| [36] | Leela NK, Sapna VP. Clove. In: Parthasarathy VA, Chempakam B, Zachariah TJ, Eds. Chemistry of Spices. 2008; pp. 146-64. [http://dx.doi.org/10.1079/9781845934057.0146] |
| [37] | Giorgio P, Mario C, Paola M, Stefania Z, Antonella D, Bruno T. Chemical composition and antimicrobial activities of essential oil of Stachys glutinosa L. from Sardinia. Nat Prod Commun 2006; 1: 1133-6. |
| [38] | Arina B, Iqbal A. In vitro fungitoxicity of the essential oil of Syzygium aromaticum. World J Microbiol Biotechnol 2012; 18(4): 317-9. |
| [39] | Giordani R, Regli P, Kaloustian J, Mikaïl C, Abou L, Portugal H. Antifungal effect of various essential oils against Candida albicans. Potentiation of antifungal action of amphotericin B by essential oil from Thymus vulgaris. Phytother Res 2004; 18(12): 990-5. [http://dx.doi.org/10.1002/ptr.1594] [PMID: 15742351] |
| [40] | Pawar VC, Thaker VS. In vitro efficacy of 75 essential oils against Aspergillus niger. Mycoses 2006; 49(4): 316-23. [http://dx.doi.org/10.1111/j.1439-0507.2006.01241.x] [PMID: 16784447] |
| [41] | Park MJ, Gwak KS, Yang I, et al. Antifungal activities of the essential oils in Syzygium aromaticum (L.) Merr. Et Perry and Leptospermum petersonii Bailey and their constituents against various dermatophytes. J Microbiol 2007; 45(5): 460-5. [PMID: 17978807] |
| [42] | Cai L, Wu CD. Compounds from Syzygium aromaticum possessing growth inhibitory activity against oral pathogens. J Nat Prod 1996; 59(10): 987-90. [http://dx.doi.org/10.1021/np960451q] [PMID: 8904847] |
| [43] | Bae EA, Han MJ, Kim NJ, Kim DH. Anti-Helicobacter pylori activity of herbal medicines. Biol Pharm Bull 1998; 21(9): 990-2. [http://dx.doi.org/10.1248/bpb.21.990] [PMID: 9781854] |
| [44] | Li Y, Xu C, Zhang Q, Liu JY, Tan RX. In vitro anti-Helicobacter pylori action of 30 Chinese herbal medicines used to treat ulcer diseases. J Ethnopharmacol 2005; 98(3): 329-33. [http://dx.doi.org/10.1016/j.jep.2005.01.020] [PMID: 15814268] |
| [45] | Betoni JE, Mantovani RP, Barbosa LN, Di Stasi LC, Fernandes J A. Synergism between plant extract and antimicrobial drugs used on Staphylococcus aureus diseases. Mem Inst Oswaldo Cruz 2006; 101(4): 387-90. [http://dx.doi.org/10.1590/S0074-02762006000400007] [PMID: 16951808] |
| [46] | Fu Y, Zu Y, Chen L, et al. Antimicrobial activity of clove and rosemary essential oils alone and in combination. Phytother Res 2007; 21(10): 989-94. [http://dx.doi.org/10.1002/ptr.2179] [PMID: 17562569] |
| [47] | Juven BJ, Kanner J, Schved F, Weisslowicz H. Factors that interact with the antibacterial action of thyme essential oil and its active constituents. J Appl Bacteriol 1994; 76(6): 626-31. [http://dx.doi.org/10.1111/j.1365-2672.1994.tb01661.x] [PMID: 8027009] |
| [48] | Farag RS. Antioxidant activity of some spice essential oils on linobic acid oxidation in aqueous media. JAOGS 1989; 66: 792-9. |
| [49] | Yajko DM, Madej JJ, Lancaster MV, et al. Colorimetric method for determining MICs of antimicrobial agents for Mycobacterium tuberculosis. J Clin Microbiol 1995; 33(9): 2324-7. [PMID: 7494021] |
| [50] | Collins L, Franzblau SG. Microplate alamar blue assay versus BACTEC 460 system for high-throughput screening of compounds against Mycobacterium tuberculosis and Mycobacterium avium. Antimicrob Agents Chemother 1997; 41(5): 1004-9. [PMID: 9145860] |
| [51] | Kumar M, Khan IA, Verma V, Kalyan N, Qazi GN. Rapid, inexpensive MIC determination of Mycobacterium tuberculosis isolates by using microplate nitrate reductase assay. Diagn Microbiol Infect Dis 2005; 53(2): 121-4. [http://dx.doi.org/10.1016/j.diagmicrobio.2005.05.011] [PMID: 16168616] |
| [52] | OBrien J, Wilson I, Orton T, Pognan F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur J Biochem 2000; 267(17): 5421-6. [http://dx.doi.org/10.1046/j.1432-1327.2000.01606.x] [PMID: 10951200] |
| [53] | Cressy HK, Jerrett AR, Osborne CM, Bremer PJ. A novel method for the reduction of numbers of Listeria monocytogenes cells by freezing in combination with an essential oil in bacteriological media. J Food Prot 2003; 66(3): 390-5. [http://dx.doi.org/10.4315/0362-028X-66.3.390] [PMID: 12636290] |
| [54] | Velluti A, Sanchis V, Ramos AJ, Egido J, Marín S. Inhibitory effect of cinnamon, clove, lemongrass, oregano and palmarose essential oils on growth and fumonisin B1 production by Fusarium proliferatum in maize grain. Int J Food Microbiol 2003; 89(2-3): 145-54. [http://dx.doi.org/10.1016/S0168-1605(03)00116-8] [PMID: 14623380] |
| [55] | Friedman M, Henika PR, Mandrell RE. Bactericidal activities of plant essential oils and some of their isolated constituents against Campylobacter jejuni, Escherichia coli, Listeria monocytogenes, and Salmonella enterica. J Food Prot 2002; 65(10): 1545-60. [http://dx.doi.org/10.4315/0362-028X-65.10.1545] [PMID: 12380738] |
| [56] | Chiaradia V, Paroul N, Cansian RL, et al. Synthesis of eugenol esters by lipase-catalyzed reaction in solvent-free system. Appl Biochem Biotechnol 2012; 168(4): 742-51. [http://dx.doi.org/10.1007/s12010-012-9814-5] [PMID: 22864649] |