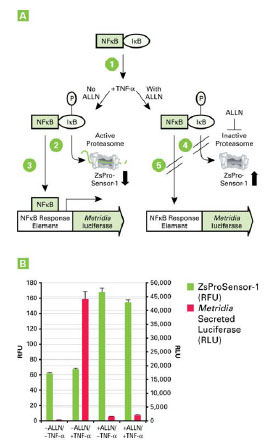
Fig. (4) Panel A. Requirement for active proteasomes in TNF-α-induced, NFκB-dependent signaling. Inactive NFκB is sequestered in the cytoplasm by IκB. IκB must be phosphorylated upon TNF-α induction (1) and degraded by the proteasome (2) in order for NFκB to translocate to the nucleus and initiate signaling (3). Alternatively, when the proteasome is inhibited by the peptide ALLN (4), IκB is not degraded and NFκB cannot translocate (5). The status of the proteasome (active or inactive) can be monitored based on ZsProSensor-1 levels. Panel B. NFκB activation by TNF-α requires proteasomal activity. High levels of Metridia luciferase signal were only observed in the absence of ALLN and the presence of TNF-α. When the proteasome is inactivated by ALLN (monitored by increasing levels of ZsProSensor-1 fluorescence), the NFκB signaling pathway cannot respond effectively to TNF-α stimulation.