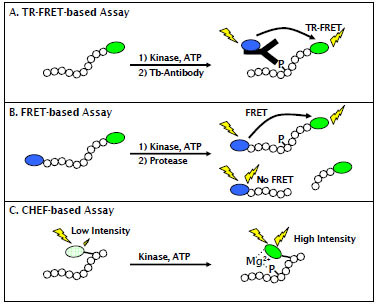
Fig. (1) Schematic of fluorescent assay formats used to characterize AMPK activators of inhibitors. (A) The TR-FRET format detects association between fluorescein labeled, phosphorylated peptide and a terbium-labeled phosphospecific antibody. (B) The FRET-based format uses a peptide substrate terminally labeled with a coumarin-fluorescein FRET pair and measures the amount of phosphorylated product due to a decrease in sensitivity of the phosphorylated peptide to proteolysis. (C) The CHEF-based format uses a peptide substrate that incorporates the non-natural, fluorogenic Sox residue. Upon phosphorylation of a proximal serine, threonine, or tyrosine residue, the Sox moiety forms a Mg2+ mediated bridge between the Sox residue and the phosphate group and becomes fluorescent.