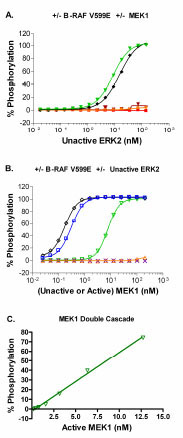
Fig. (3) (A) Determination of the amount of the unactivated downstream kinase (ERK2) that leads to complete phosphorylation of the peptide substrate in the presence of: No B-RAF V599E and 200 nM active MEK1 (▼, double cascade); 150 nM (10 µg/ml) active B-RAF V599E and 200 nM unactivated MEK1 (♦, triple cascade); No B-RAF V599E and No MEK1 (■); No B-RAF V599E and 200 nM unactivated MEK1 (•); or 150 nM (10 µg/ml) active B-RAF V599E and No MEK1 (▼). (B) Determination of the amount of activated MEK1 that leads to complete phosphorylation of the peptide substrate in the presence of: No B-RAF V599E and 140 nM unactivated ERK2 (▽, Double cascade); ~150 nM active B-RAF V599E and 140 nM unactivated ERK2 (□); ~150 nM active B-RAF V599E and No ERK2 (×). Determination of the amount of unactivated MEK1 that leads to complete phosphorylation of the peptide substrate in the presence of: ~150 nM active B-RAF V599E and 140 nM unactivated ERK2 (◇, triple cascade); No B-RAF V599E and 140 nM unactivated ERK2 (Δ). (C) Linear plot of the percent phosphorylation achieved for the double cascade with increasing concentration of active MEK1, using the optimized concentration for unactive ERK2 (140 nM), with R2 ≥ 0.99. All data points represent the mean ± standard deviation from triplicate samples.