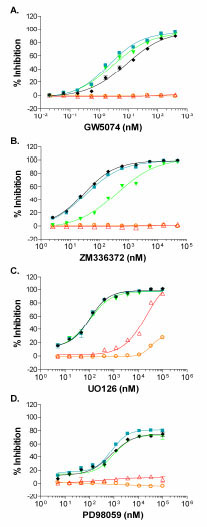
Fig. (6) Inhibitor titrations are shown for the FRET assays: ERK2 direct (O); MEK1-ERK2 double cascade (Δ); B-RAF-MEK1-ERK2 triple cascade (▼); B-RAF V599E-MEK1-ERK2 triple cascade (■); and C-RAF-MEK1-ERK2 triple cascade (♦). For all assay formats, concentrations of active and unactivated enzymes were optimized in order to achieve 10 - 50% phosphorylation of the FRET substrate in the presence of 100 µM ATP and the absence of inhibitor. Two of the compounds tested are known to inhibit RAF activity, GW5074 (A) and ZM336372 (B). The other two compounds are allosteric MEK inhibitors: U0126 (C) can differentially inhibit MEK activity as well as MEK activation, while PD98059 (D) inhibits RAF phosphorylation of MEK but not MEK activity. All data points represent the mean ± standard deviation from quadruplicate samples.