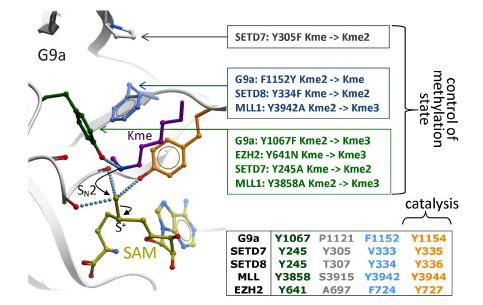
Fig. (7) Catalysis and control of methylation specificity. Backbone carbonyl oxygens and a catalytic tyrosine (orange) surrounding the
departing methyl group of SAM (yellow) favor a nucleophilic attack by the ε-nitrogen of the substrate lysine (magenta), which must have
been de-protonated beforehand [8]. A limited number of residues (green, gray, cyan) restrict the alignment of a lone-pair on the accepting
nitrogen with the scissile sulfur-methyl bond, a geometry necessary for methyl transfer to occur. Mutational analyses reported for G9a [18,
33], SETD7 [14, 37], SETD8 [34], MLL [21] and somatic mutations reported in EZH2 [35] confirm that these residues control the final
methylation product: mono-, di- or tri-methyl lysine (Kme, Kme2, or Kme3 respectively).