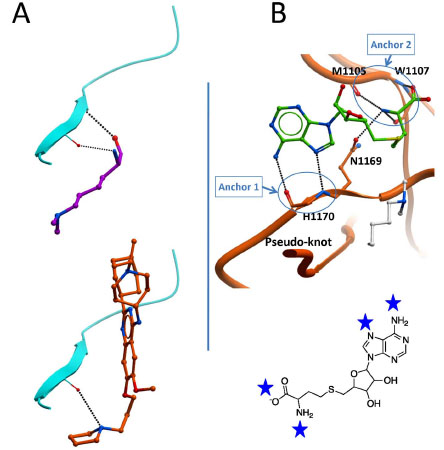
Fig. (9) Conserved interactions at the peptide and cofactor sites.A- A double hydrogen bond observed between the backbone of the
substrate lysine (magenta) and the I-SET domain (cyan) is present in all SET domain PMT ternary complexes (top), and is partially recapitulated
by the dimethylamine group of the potent inhibitor UNC0638 (bottom, orange). B- Six hydrogen bonds with the co-crystallized cofactor
(or the analog synefungin) are observed with backbone atoms and a conserved asparagine side-chain of the SET domain across all available
structures. This likely points at strong interaction potentials (blue stars) within the SAM pocket, clustered at two anchor points, that
should be targeted by chemical inhibitors.