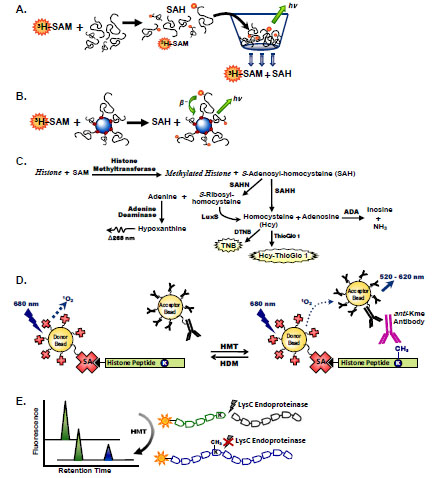
Fig. (1) Histone methyltransferases assay formats. Filter binding assays to measure histone methyltransferase activity utilize radiolabeled SAM to measure transfer of tritiated methyl groups to histone substrates (A). Unreacted 3H-SAM is removed from reaction solutions via filtration through a vacuum plate. Addition of scintillation fluid permits quantitation of radiolabeled products retained on the filter plate. (B) SPA methyltransferases assays utilize beads containing scintillation fluid that emit light when excited by β-particles released from radioactive decay of bead-bound substrates. (C) Coupled enzyme assays measure histone methyltransferase activity by transformation of SAH to products that can be measured by fluorescence or UV/Vis spectroscopy via Hcy or S-ribosyl-homocysteine intermediates. (D) Methylation of a biotinylated histone peptide by AlphaScreen is measured using streptavidin-coated donor beads and anti-immunoglobulin–conjugated acceptor beads in the presence of a specific antibody raised against the methylated lysine product. Transfer of singlet oxygen from laser-excited donor beads to acceptor beads in close proximity results in a chemiluminescent signal. (E) The LysC endoproteinase distinguishes between methyltransferase substrate and product peptides by selective proteolysis at unmodified lysine residues. Electrophoretic separation of tagged fluorescent peptides permits quantitation of enzymatic activity following proteolysis with LysC.