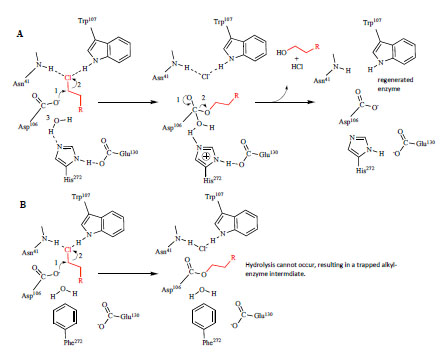
Fig. (1) Catalytic mechanism of dehalogenation by DhaA and strategy for trapping the covalent intermediate. A. In the first step of
catalysis, the nucleophile, Asp106 attacks the α-carbon of the chloroalkane (shown in red) to produce a covalent, alkyl-enzyme intermediate.
His272 catalyzes hydrolysis of the intermediate, the release of products from the active site, and regeneration of enzyme. Glu130 provides
structure at the active site and stabilizes the positive charge on His272 that forms during hydrolysis. In addition to forming the halide binding
site, Trp107 and Asn41 stabilize the Cl- leaving group following hydrolysis [16]. B. The strategy for trapping the covalent intermediate was to
replace His272 with a residue (e.g. Phe) that cannot act as a general base, and therefore cannot hydrolyze the alkyl-enzyme intermediate.