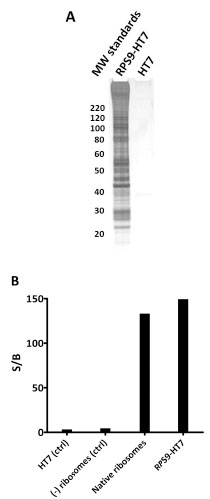
Fig. (12) Capture of intact 80S ribosome from HEK-293T cells
using RPS9-HT7. A. Overexpressed RPS9-HT7 (or HT7 alone,
control) was captured to HaloLink resin and treated with TEV protease
to release RPS9 and its interacting partners. The eluted samples
were analyzed by SDS-PAGE and silver staining. Mass analysis
of the same samples verified the following was present: 31 of 33
40S proteins, 42 of 50 60S proteins, 2 poly-A binding proteins, 1
GNF exchange protein, 9 nuclear ribonucleoproteins, 2 initiation
factors, 2 elongation factors, and 2 splicing factors. For a complete
list see Table S7. BIn vitro luciferase translation assay showing
activity of ribosomes isolated via RPS9-HT7. RPS9-HT7 was transiently
expressed in HEK-293T cells stably expressing Fluc
mRNA. Ribosomes were isolated via RPS9-HT7 and released using
TEV protease. HT7 alone and untransfected cells were processed in
the same manner as negative controls. Signal to background calculations
indicated the generation of active luciferase from the RPS9-
HT7 complex isolation but not from the negative controls. Commercially
available native ribosomes, included as a positive control,
were also able to generate active luciferase in vitro.