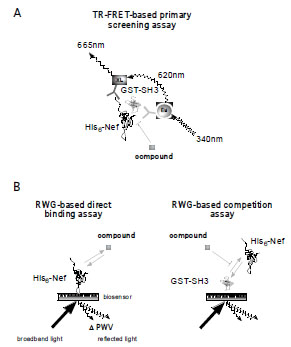
Fig. (1A) Principle of a TR-FRET-based Nef/SH3 protein-protein
interaction assay. The quaternary complex of His-Nef, GST-SH3,
α-GST Eu-labeled mAb, and α-His XL665-labeled mAb generates
the proximity for a FRET pair between the donor lanthanide
fluorophor europium and acceptor fluorophor XL665. The Eu
chelates emit light at 620 nm after excitation at 340 nm and thereby
excite neighboring XL665 FRET acceptors. Excited XL665 decays
under the emission of photons with a wavelength of 665 nm. The
665 nm emission or the 665/620 nm ratio is proportional to the
number of quaternary complexes formed. Excited Eu chelates have
a long half-life. The time-resolved detection minimizes interference
with extrinsic fluorescence. (B) Schematic of the resonant
waveguide grating (RWG)-based assay for monitoring proteinligand
or protein-protein interactions in real time. Here, the target
protein His-Nef (or off-target GST-SH3) is immobilized on the preactivated
aldehyde surface of an optical biosensor whose photonic
crystal composition allows the reflection of broadband light only in
a narrow range of wavelengths, the peak wavelength value (PWV).
Binding of a chemical (compound) or biological (protein) ligand to
His-Nef results in a change of the refractory index of the biosensor,
measured as a shift of the peak wavelength value (ΔPWV) that is
proportional to the change in mass on the surface.