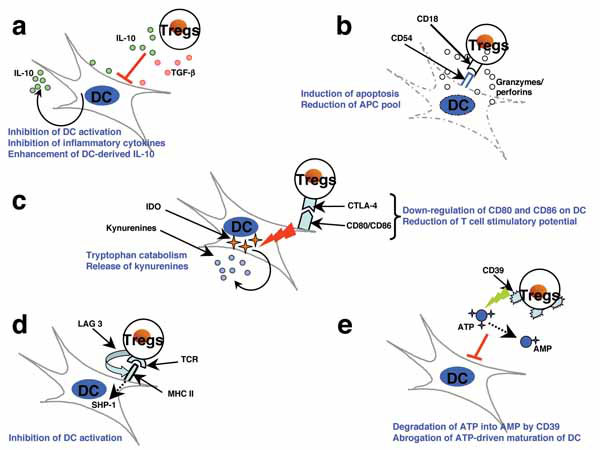
Fig. (2) Several mutually nonexclusive mechanisms have been proposed to account for the modulation of DCs by Tregs. The suppression of DCs by Tregs is mediated by both soluble factors and cell-associated molecules. However, recent reports suggest that CTLA-4 molecule has a key role in Treg-mediated suppressive functions. (a) Treg-derived IL-10 and TGF-β induce a suppressive phenotype in DCs and inhibit inflammatory cytokine expression by DCs while enhancing the secretion of the anti-inflammatory cytokine IL-10. (b) Activated Tregs express high levels of granzyme A and perforin and exert CD18/CD54 adhesive interaction-dependent cytotoxicity against both immature and mature DCs, thus reducing the antigen-presenting and co-stimulatory pool. (c) CTLA-4 interaction with B7 molecules transduces a negative signal for DCs CTLA-4 diminishes the co-stimulatory molecules CD80 and CD86 and converts DCs into less potent APCs with reduced T cell stimulatory potential. CTLA-4 can also induce indoleamine 2,3-dioxygenase (IDO) expression in DCs. IDO catalyzes the conversion of tryptophan into kynurenines, which are potent immunosuppressive metabolites. (d) Tregs can interact with MHC class II on DCs via lymphocyte activation gene-3 (LAG-3), a CD4-related transmembrane protein that inhibits DC activation by a mechanism involving ERK-mediated recruitment of SHP-1. (e) Tregs express CD39 (nucleoside triphosphate diphosphohydrolase-1), an ectoenzyme that degrades ATP to AMP. Thus, Tregs reduce ATP-mediated activation of DCs and the adjuvant activities of ATP. Some of these mechanisms are also reported to be critical in Treg-mediated regulation of functions of macrophages and B cells. The figure is reprinted from Am J Pathol 2009, 174:1575-1587 (Ref. [31]) with permission from the American Society for Investigative Pathology.