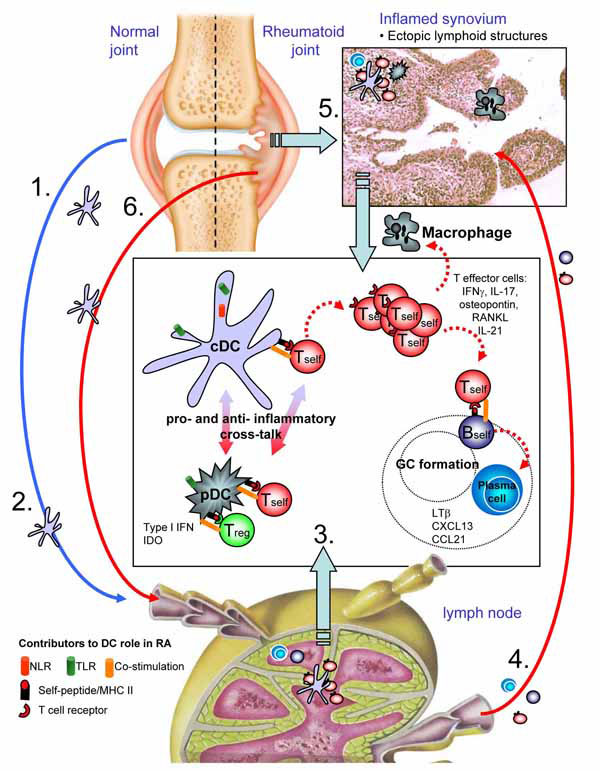
Fig. (1) DC biology in arthritic disease. Steady state migration of cDCs derived from articular environments (1) could result in presentation
of joint relevant antigens in the lymph node (LN). Under normal circumstances this would function to maintain tolerance, but aberrant activation
of cDCs through stimulation of pattern recognition and/or innate receptors (such as TLRs, NLRs) resulting from infection or mechanical
damage (2) prime self-restricted T cells that have escaped thymic selection. Presentation of self-antigens by cDCs expressing high levels of
costimulatory molecules such as CD40 would promote proliferation of autoreactive T cells (3). Acquisition and presentation of self antigens
by pDCs may function in two ways, in some ways potentiating inflammation through type I interferon production but equally regulating
autoreactive T cell function through recruitment of regulatory T cells and/or secretion of IDO. Adoption of proinflammtory effector function
of autoreactive T cells is likely imparted by cytokine signals derived from arthitogenic DCs. In the LN, these effector functions facilitate
helper activity for self-restricted B cells, supporting immunoglobulin class switching and plasma cell differentiation, culminating in high titres
of auto-antibodies. Self restricted T effectors recirculating to joints encounter APCs in the now inflamed tissue (4). DCs will re-activate these
arthritogenic T cells where their effector functions support macrophage activation, expression of costimulaoty molecules such as RANKL and
stimulate joint pathology (5). With increasing joint damage, further antigen is released, perpetuating disease in the LN (6), and with chronicity,
facilitating development of ectopic lymphoid foci in synovial tissue. The processes occurring the LN to initiate disease can now occur in
the tissue itself, adding to the self-perpetuating and chronic nature of this severely disabling autoimmune disorder. Images courtesy of the
Wellcome Library.