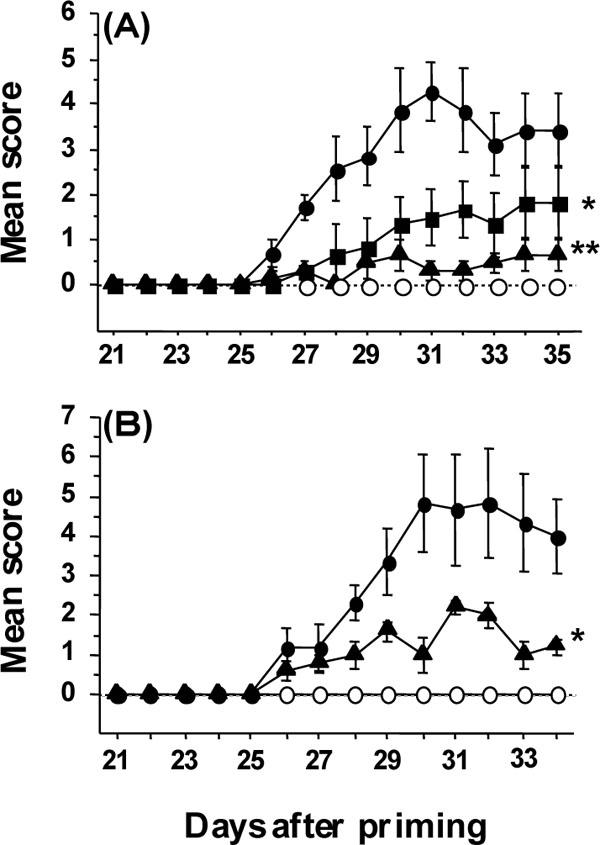
Fig. (3) (A) Prophylactic and therapeutic protection against CIA by
AMC. AMC at 0 (●), 3 (■), or 30 mg/kg (▲) was orally administered
to immunized mice (n = 7 or 8 per group) after priming as
described in Materials and Methods. Mock-immunized mice (○)
were administered 5% gum arabic. Progression scores of diseases
are expressed by the mean score±SEM of mice in a group. *p<0.02
and **p<0.002 vs. AMC at 0 mg/kg by RM-ANOVA. (B) Therapeutic
protection against collagen-induced arthritis by AMC. AMC
at 0 (●) or 30 mg/kg (▲) was therapeutically administered to immunized
mice (n = 7 or 8 per group) from day 27 after the onset of
arthritis as described in Materials and Methods. Mock-immunized
mice (○) were administered 5% gum arabic. The development of
arthritis is expressed by the mean score±SEM. *p<0.03 vs. AMC at
0 mg/kg by RM-ANOVA.