- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

The Open Bioactive Compounds Journal
(Discontinued)
ISSN: 1874-8473 ― Volume 9, 2020
Preparation, Characterization and Antimicrobial Activity of Schiff Base of (E) - N - (4-(Thiophen-2-ylmethyleneamino) Phenylsulfonyl) Acetamide Metal Complexes
Ahmad S. Abu-Khadra1, *, Ahmed S. Afify2, Amr Mohamed3, 4, Rabie S. Farag5, Hassan Y. Aboul-Enein6, *
Abstract
Aim:
Metal complexes of (E)-N-(4-(thiophen-2-ylmethyleneamino) phenylsulfonyl) acetamide (S.TH) Schiff bases derived from sulfacetamide (N-[4-(amino-phenyl) sulfonil] acetamide) and 2-thiophenecarboxaldehyde were synthesized and characterized.
Methods and Results:
The synthesized compositions have been characterized using different physico-chemical techniques. The investigation included elemental analysis, melting point measurements, proton NMR, UV spectroscopy, FT-IR, magnetic susceptibility, conductance measurements, mass spectral analysis, and inductively coupled plasma mass spectrometry (ICP-MS) for determining the concentrations of metal ions. The measured values for molar conductance indicated that the majority of the prepared complexes were nonelectrolytes. The biological activity of the prepared compositions has been investigated.
Conclusion:
Spectroscopic studies suggested that most of the complexes were coordinated in a regular octahedral arrangement where S.TH ligand and the central metal atom were coordinated through two N amino azomethine groups (−HC=N−) and two sulfur atoms of S thiophene rings in 2L:1M molar ratio. Complexes have shown a promising activity upon screening for the antibacterial characteristics, and antifungal (Aspergillus fumigates and Candida albicans)
Article Information
Identifiers and Pagination:
Year: 2018Volume: 6
First Page: 1
Last Page: 10
Publisher Id: TOBCJ-6-1
DOI: 10.2174/1874847301806010001
Article History:
Received Date: 07/12/2017Revision Received Date: 27/01/2018
Acceptance Date: 29/01/2018
Electronic publication date: 21/02/2018
Collection year: 2018
open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: (https://creativecommons.org/licenses/by/4.0/legalcode). This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
* Address correspondence to this author at the Pharmaceutical and Medicinal Chemistry Department, National Research Centre, Pharmaceutical and Drug Industries Research Division, Professor Hassan Y. Aboul-Enein, Cairo, Egypt; Tel:+201003678948; E-mails: haboulenein@yahoo.com, ahmad.sabrry@su.edu.eg
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 07-12-2017 |
Original Manuscript | Preparation, Characterization and Antimicrobial Activity of Schiff Base of (E) - N - (4-(Thiophen-2-ylmethyleneamino) Phenylsulfonyl) Acetamide Metal Complexes | |
1. INTRODUCTION
Schiff bases (imines) are the products of condensation of primary amines with carbonyl compounds in acidic medium. Imines were first reported in 1864 by Schiff H [1Schiff H. Mittheilungen aus dem Universitätslaboratorium in Pisa: Eine neue Reihe organischer Basen. Justus Liebigs Ann Chem 1864; 131(1): 118-9.
[http://dx.doi.org/10.1002/jlac.18641310113] ]. The formation of carbon-nitrogen double bonds is a very important step in organic synthesis due to their significant biological activities such as anticancer [2Popp FD, Kirsch W. Synthesis of potential anticancer agents V. schiff bases and related compounds1-2. J Org Chem 1961; 26(10): 3858-60.
[http://dx.doi.org/10.1021/jo01068a056] ], anti-inflammatory and antitumor agents [3Hadjipavlou-Litina DJ, Geronikaki AA. Anti-inflammatory activity of some novel 1-[3-(aroylo)] and one 1-[3-(aryloxy)]-propyl aminothiazole in correlation with structure and lipophilicity. Arzneimittelforschung 1996; 46(8): 805-8.
[PMID: 9125283] ], antibacterial, insecticidal, antimicrobial [4Wadher SJ, Karande NA, Yeole PG. Synthesis and biological evaluation of schiff base of dapsone and their derivative as antimicrobial agents. Int J Pharm Tech Res 2009; 1(1): 12.], anticonvulsant activity [5Cates LA, Rashed MS. Phosphorus GABA analogues as potential prodrugs. Pharm Res 1984; 1(6): 271-4.
[http://dx.doi.org/10.1023/A:1016350119870] [PMID: 24277362] ], antituberculosis [6Solak N, Rollas S. Synthesis and antituberculosis activity of 2-(aryl/alkylamino)-5-(4-aminophenyl)-1, 3, 4-thiadiazoles and their Schiff bases. ARKIVOC 2006; 12: 173-81.]. Additionally, Schiff bases are useful as versatile constituents in nucleophilic addition reactions including organometallic reagents [7Voronkov MG, Trukhina AA, Vlasova NN. Acyl iodides in organic synthesis: II. Reactions with acyclic and cyclic ethers. Russ J Org Chem 2002; 38(11): 1579-81.
[http://dx.doi.org/10.1023/A:1022545630736] ] as well as in the reactions including cycloadditions [8Taggi AE, Hafez AM, Wack H, Young B, Ferraris D, Lectka T. The development of the first catalyzed reaction of ketenes and imines: catalytic, asymmetric synthesis of β-lactams. J Am Chem Soc 2002; 124(23): 6626-35.
[http://dx.doi.org/10.1021/ja0258226] [PMID: 12047183] ]. They have been widely used in dye and food industries, catalytic processes, analytical chemistry, in addition to several agrochemical applications [9Tsuge O, Kanemasa S. Recent advances in azomethine ylide chemistry InAdvances in heterocyclic chemistry Jan 1 Academic Press 1989; 45(23): 231-349.
[http://dx.doi.org/10.1016/S0065-2725(08)60332-3] ].
The Schiff base produced from the reaction of 2,5-thiophenedicarboxaldehyde with o-amino benzenethiol is 2,5-bis(benzothiazolidin-2-yl)thiophene(I) which is able to react with Pb(II), Cd(II), Ag(I), Zn(II) and Cu(II) forming different complexes [10Salameh AS, Tayim HA. Reaction of 2, 5-(Dibenzothiazolin-2-yl) thiophene with Some metal ions. Polyhedron 1983; 2(8): 829-34.
[http://dx.doi.org/10.1016/S0277-5387(00)87213-7] ]. The condensation of 2-thiophenecarboxaldehyde with o-amino thiophenol gives 2-thiazolin-2-ylthiophene, which reacts with Pd(II), Cd(II), Pb(II), Cu(II), Ni(II), Ag(I) Zn(II) forming new complexes [11Tayim HA, Salameh AS. Reactions of 2-thiazolin-2-ylthiophene with some metal ions. Polyhedron 1983; 2(10): 1091-4.
[http://dx.doi.org/10.1016/S0277-5387(00)81461-8] ]. Several methods can be utilized for determining the transition metal ions. These methods include: Ion Chromatography (IC), Inductively Coupled Plasma Mass Spectrometry (ICP-MS), Atomic Absorption Spectroscopy (AAS), and Inductively Coupled Plasma Atomic Emission Spectrometry (ICP-AES). The concurrent multi-element determination with AAS is not possible, while ICP-MS and ICP-AES techniques are more suitable. The ICP-MS technique is chosen due to its capability of mainly detecting metals and several nonmetals. ICP-MS has several advantages when compared to the other analytical techniques. This includes a higher throughput and better detection limits for most elements than Graphite Furnace Atomic Absorption Spectroscopy (GFAAS). It can also handle both complex and simple matrices with minimum matrix interference due to the high temperature of ICP source, in addition to its ability to obtain isotropic information [12Cartwright AJ, Jones P, Wolff JC, Evans EH. Detection of phosphorus tagged carboxylic acids using HPLC-SF-ICP-MS. J Anal At Spectrom 2005; 20(2): 75-80.
[http://dx.doi.org/10.1039/b415962d] ].
The aim of this study is to prepare, characterize and elucidate the geometrical structures of new sulfactamide Schiff bases metal complexes and to investigate some of their biological activity.
2. EXPERIMENTAL
2.1. Materials and Methods
All solvents and chemicals used were of extra-pure grade. Sulfacetamide samples were obtained from EPICO Pharm (Cairo, Egypt). Melting points were denoted using (BI Bamtead Electothermal). TLC was visualized using VL-6-LC UV lamp (VILBER, Germany). The elemental analysis for (C, H, N, S) was carried out using Fisons EA 1108 CHNS Micro Analyzer (Thermo Scientific, USA). Metal ions concentrations were measured using ICP Perkin Elmer/Optima 7000. The UV spectra have been recorded using UV-Vis spectrophotometer (UV-1700, Shimadzu). Measurements of molar conductance were carried out in DMF at 25˚C ±5˚C using a conductivity bridge (model 305, Systronics Ltd., India). Magnetic susceptibility for powdered samples was measured using a magnetic susceptibility balance (Sherwood, UK). The diamagnetic corrections were done by Pascal's constant and Hg[Co(SCN)4] was used as a calibrant. Mass spectral analysis was carried out using Shimadazu Qp-2010 Plus. Infrared spectra were recorded using a Perkin-Elmer FT-IR 1650 spectrophotometer in wave number region 4000-200 cm-1 as KBr pellet. The proton NMR spectra were recorded using Mercury-300 bb “NMR 300 MHz”, using DMSO as a solvent and tetramethylsilane (TMS) as an internal standard. Antibacterial and antifungal activities were performed sing the diffusion agar technique [13Bauer AW, Kirby WM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol 1966; 45(4): 493-6.
[http://dx.doi.org/10.1093/ajcp/45.4_ts.493] [PMID: 5325707] ] and then, the sensitivity of clinically pathogenic microorganisms to antibiotics and other sulfacetamide complexes was determined by the assay plates using 100 μl (5 mg/ml concentration) of the tested samples. The plates were incubated at 28°C for 3 days (for fungi) and at 37°C for 1 day (for bacteria).
2.2. Synthesis of Schiff Base (S.TH) Ligand
The ligand was prepared by adding a hot ethanolic solution of 2-Thiophene carboxyaldehyde (0.03 mole) to a hot ethanolic solution of (0.03 mole) sulfacetamide in 1:1 molar ratio, with vigorous stirring. The reaction mixture was refluxed for 4 hours till a yellow precipitate was formed, which was then washed several times, recrystallized from ethanol, and dried in a vacuum over anhydrous calcium chloride. The purity was checked by TLC and by melting point measurement.
S.TH Ligand had a pale yellow color (melting point 206-208°C) yield 84.6%, molecular weight 308.38 g/mol with molecular formula C13H12N2O3S2. The elemental analysis was as follows: C 50.63 (51.03), H 3.92 (3.98), N 9.08 (9.21), S 20.80 (20.77).
The reaction can be expressed as shown in Fig. (1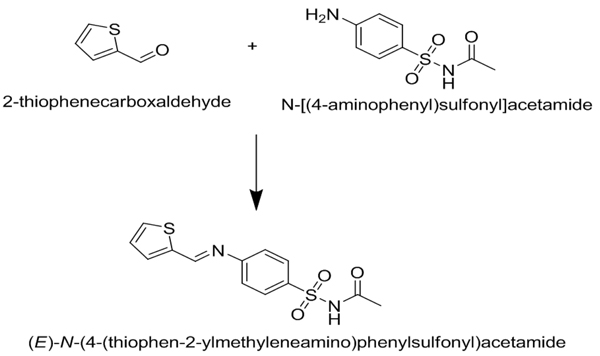 ):
):
 |
Fig. (1) Condensation reaction between 2-thiophenecarboxyaldehyde and sulfacetamide give (E)-N-(4-(thiophen-2-ylmethyleneamino) phenylsulfonyl)acetamide S.TH. |
2.3. Synthesis of the Schiff Base Metal Complexes:
The Schiff base metal complexes were prepared by adding a hot ethanolic (or distilled water) solution of metal salts (0.03 mole) with vigorous stirring to a hot ethanolic solution of (E)- N- (4- (thiophen-2-ylmethyleneamino) phenylsulfonyl) acetamide S.TH (0.06 mole) in 1M:2L molar ratio (where M is noted for the metal and L is for the ligand). Silver complex was prepared in equimolar ratio to the Schiff base. The reaction was refluxed for several hours until a colored (or sometimes white) precipitate is formed, which was then washed several times and recrystallized from ethanol (or distilled water) then dried in a vacuum over anhydrous calcium chloride. The purity was checked by TLC and by melting point.
2.4. Inductively Coupled Plasma Mass Spectrometry (ICP-MS)
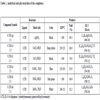
5 ml of the internal standard were transferred to a test tube (the internal standard consists mainly of deionized water, with hydrochloric of nitric acid, and indium and/or gallium, depending on the type of sample), along with 10–500 µL of the metal complex sample. The mixture was then shaken by vortex, and after that the mixture was loaded into the instrument’s auto-sampler tray. The instrument’s operating conditions are listed in Table 1.
3. RESULTS AND DISCUSSION
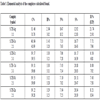
Most of the prepared complexes were colored, stable at room temperature for extended periods, decomposed on heating and insoluble in water but readily soluble in strong coordinating solvents like DMF and DMSO (S.TH Co 2-1 complex was readily soluble in water) (Table 2).
3.1. Elemental Analyses of the Complexes
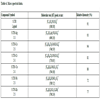
The results of elemental analyses are shown in Table 3, which are in good agreement with those required by the proposed formula (calculated). Inductively coupled plasma (ICP-MS) technique plays an important role in confirming the structure of the complexes by determining metal ion concentration. The metal percentages of newly synthesized Schiff base metal complexes shown in Table 3 give a good agreement with the proposed formula (calculated), and confirm binding of the metal ion to ligand sites.
3.2. Infrared Spectra
The nature and functional groups attached to metal atoms can be provided by FTIR spectra. The IR spectra of the complexes were compared with those of their ligand (S.TH) and the previously reported complexes [14Blasco FL, Perelló J, Latorre J. Borrás, S. Garciá-Granda, Cobalt(II), Nickel(II), and Copper(II) complexes of sulfanilamide derivatives: Synthesis, spectroscopic studies, and antibacterial activity. Crystal structure of. J Inorg Biochem 1996; 61(2): 143-54. [Co(sulfacetamide)2(NCS)2].
[http://dx.doi.org/10.1016/0162-0134(95)00053-4] [PMID: 8576708] ]. The spectra of compounds have numerous bands, as shown in Table (4), which summarizes the main bands and their proposed groups. The absence of IR characteristic bands for amino group of aryl amine and the carbonyl group of aldehyde is an indication of forming Schiff base compounds. This is further confirmed as shown in the equation of Fig. (1 ) by the appearance of a new characteristic band of νC=N at 1578 cm-1, which has already shifted to higher frequencies in all complexes suggesting that this group is involved in the coordination with metal [15Agarwal PB, Kumar R, Aslam V, Khan A. Synthesis, physico-chemical and thermal characteristics of seven and ten coordinated complexes of trivalent lanthanides derived from 4[n-(cinnamalidene) amino] antipyrine semicarbazone and diphenyl sulfoxide. J Iran Chem Soc 2007; 4(1): 12.
) by the appearance of a new characteristic band of νC=N at 1578 cm-1, which has already shifted to higher frequencies in all complexes suggesting that this group is involved in the coordination with metal [15Agarwal PB, Kumar R, Aslam V, Khan A. Synthesis, physico-chemical and thermal characteristics of seven and ten coordinated complexes of trivalent lanthanides derived from 4[n-(cinnamalidene) amino] antipyrine semicarbazone and diphenyl sulfoxide. J Iran Chem Soc 2007; 4(1): 12.
[http://dx.doi.org/10.1007/BF03245809] ]. The IR spectrum of S.TH exhibits many potential donor sites like nitrogen of the azomethine group (C=N), sulfonamide oxygen (SO2), nitrogen of secondary amine (NH), O acetamido C=O, and sulfur atom of S thiophene ring. The asymmetric and symmetric (SO2) group stretching vibrations in the sulfonamides were observed at 1331-1325 and 1157-1152 cm-1, respectively, which indicate no significant shifts. This behavior has been observed in similar sulfonamide complexes that do not coordinate through this group. The band of the carbonyl group (C = O) appears around 1717-1709 cm-1, has nearly not changed, indicating that they are also not involved in coordination. The hydrated complexes exhibited an IR bands approximately around 3447 cm-1 range due to ν(H2O) bands, which refer to water molecules coordinated to the metal in the complexes. The coordination of metals to ligand potential sites is further supported by appearance of non-ligand bands around 519-502 cm-1 and around 490-450 cm-1 due to υM_N and υM_S, respectively. Studies on the stretching vibrations of these bonds are important in elucidating the structure of the complex [16Moustafa M. Spectrophotometric analysis, thermal analysis and gravimetric determination of some metal ions with oxime and Schiff’s base derivatives of N-furoylphenylhydroxylamine. J Therm Anal Calorim 1997; 50(3): 463-71.
[http://dx.doi.org/10.1007/BF01980506] ]. Other bands like υC-H Ar appears around 3050 cm-1 and υC-H Al appears around 2800 cm-1, other bands such as C=C appears around 1490 cm-1.
3.3. Molar Conductivity Measurements
The molar conductance of the complexes (Λm) can be calculated using the relation: Λm= K/C, where K is specific conductivity of the complex, and C is the molar concentration of the metal complex solutions. The chelates were dissolved in DMF and the molar conductivities of 10-4 M of their solutions at 25 ± 2°C were measured. Table (5) shows that low molar conductivity values of the complexes (Λm = 11.67-33.27 Ω-1mol-1 cm2) which indicate that these complexes are non-electrolytes [17Mohamed GG, Omar MM, Hindy AM. Metal complexes of Schiff bases: preparation, characterization, and biological activity. Turk J Chem 2006; 30(3): 361-82.]. Except Co(II) and Cr(III) complexes, they have high molar conductance of 120.21 and 114.61 Ω−1mol-1cm2, respectively, which indicating the electrolytic nature of the complex [18El-Gahami MA, Abdalla EM. Synthetic and spectroscopic studies of polymeric metal complexes involving 1, 4-[5-Disulphonyl-8-Hydroxyquinoline] piperazine. J Inorg Organomet Polym 2003; 13(1): 41-8.
[http://dx.doi.org/10.1023/A:1022900914224] ].
3.4. Magnetic Susceptibility Measurements
The magnetic moment values in Table (5) show that the S.TH-Co (II) complex has magnetic moment value 5.24 B.M. which agrees well with the range normally observed for octahedral cobalt(II) complexes [14Blasco FL, Perelló J, Latorre J. Borrás, S. Garciá-Granda, Cobalt(II), Nickel(II), and Copper(II) complexes of sulfanilamide derivatives: Synthesis, spectroscopic studies, and antibacterial activity. Crystal structure of. J Inorg Biochem 1996; 61(2): 143-54. [Co(sulfacetamide)2(NCS)2].
[http://dx.doi.org/10.1016/0162-0134(95)00053-4] [PMID: 8576708] ]. The S.TH Cr (III) complex shows a magnetic moment corresponding to 3 unpaired electrons 3,71 B.M., similar to those observed in octahedral geometry [19Dobrzanska L, Wrzeszcz G, Grodzicki A, Rozploch F. Synthesis and properties of thiocyanato-bridged chromium (III)-copper (II) hydroxo complexes. Pol J Chem 2000; 74(7): 1017-22.]. The value of magnetic moment of S.TH Cu(II) complex was 2.11 B.M. which is in a good agreement with the characteristics of mononuclear, (d9, 1 unpaired electron) distorted octahedral geometry due to Jahn–Teller effect. This phenomenon is very common in six-coordinate copper (II) complexes. The d9 electronic configuration of this ion gives three electrons in the two degenerate eg orbitals, leading to a doubly degenerate electronic ground state. Such complexes distort along one of the molecular four fold axes (always labeled the z axis), which has the effect of removing the orbital and electronic degeneracies and lowering the overall energy. When such an elongation occurs, the effect is to lower the electrostatic repulsion between the electron-pair on the Lewis basic ligand and any electrons in orbitals with a z-component, thus lowering the energy of the complex where inversion center is preserved after the distortion [20Janes R, Moore EA. Metal-ligand bonding 2004.].
In case of S.TH Ni complex, the magnetic moment value is 3.04 B.M. (d8, 2 unpaired electrons, octahedral geometry) [21Gudasi KB, Patil MS, Vadavi RS, Shenoy RV, Patil SA, Nethaji M. X-ray crystal structure of the N-(2-hydroxy-1-naphthalidene) phenylglycine Schiff base. Synthesis and characterization of its transition metal complexes. Transition Met Chem 2006; 31(5): 580-5.
[http://dx.doi.org/10.1007/s11243-006-0031-3] ]. The magnetic moments values of diamagnetic Ag and Zn complexes show no d-d bands as expected for a d10 system.
3.5. Electronic Spectra
Electronic spectra of complexes can provide valuable information related to stereo chemistry of metal ions in the complexes based on the positions and number of d–d transition peaks. The electronic spectra for all compounds were obtained in DMSO solution and show absorption bands in three distinct regions as can be seen in Table (5). The first region ranging from 200 to approximately 249 nm, is characteristic for the electronic inter-ligand π→π* transitions correspond to transition C=C of the phenyl group, while the second characteristic wavelength in the region of 280 nm to approximately 350 nm is the second inter ligand n→π transition C=O, the third distinct region ranging from 410 nm to approximately 540 nm is an indicator for the Ligand to Metal Charge Transfer (LMCT) from the nitrogen atom to the transition metal center [22Chen W, Li Y, Cui Y, Zhang X, Zhu HL, Zeng Q. Synthesis, molecular docking and biological evaluation of Schiff base transition metal complexes as potential urease inhibitors. Eur J Med Chem 2010; 45(10): 4473-8.
[http://dx.doi.org/10.1016/j.ejmech.2010.07.007] [PMID: 20691510] -24Raman N, Jeyamurugan R, Senthilkumar R, Rajkapoor B, Franzblau SG. In vivo and in vitro evaluation of highly specific thiolate carrier group copper(II) and zinc(II) complexes on Ehrlich ascites carcinoma tumor model. Eur J Med Chem 2010; 45(11): 5438-51.
[http://dx.doi.org/10.1016/j.ejmech.2010.09.004] [PMID: 20864225] ]. The UV bands of SO2 group merge to form a single strong absorption band around 260 nm. Many bands positions are considerably shifted from their original positions in their ligands to blue or red regions in all complexes indicating coordination to the metal, while new bands were observed in the visible region for the complexes due to d→d transitions (Table 5). In case of a d10 system (Ag & Zn complexes) the present diamagnetic complexes show no d-d transition in the visible region.
3.6. Mass Spectra Analysis
The mass spectra of complexes are shown in Table (6). The molecular ion peaks are in a good agreement with their empirical formula as indicated from the elemental analyses. The other peaks represent fragments of the molecular ions.
3.7. Proton NMR Spectra
The proton NMR for S.TH and the diamagnetic complexes (S.TH-Ag and S.TH-Zn) are shown in Table 7. All the protons were found as expected. The spectra of the complexes were examined in comparison with their ligand. The ligand and the diamagnetic complexes give a duplet signals in the region 7.12-7.91 ppm (d, m) for the aromatic protons. Singlet peaks appeared in the region of 1.82-1.85 ppm was attributed to the methyl group. The N-H proton give a singlet signals in the region 11.85-11.91 ppm which nearly not changed compared to ligand, this indicate NH group is not involved in the coordination of metal complexes. Signals for the methine protons of the azomethine group, were observed between 8.91 and 8.80 ppm, the coordination of azomethine nitrogen is confirmed by down field shift. These numbers of protons results give a good agreement with CHNS calculated and found results.
3.8. The Suggested Structure of Complexes
Based on the previous results, we can propose these two structures for the synthesized complexes Fig. (2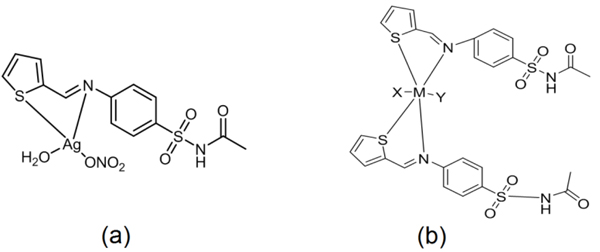 ).
).
 |
Fig. (2) (a) Proposed structure for S.TH - Ag complex, and (b) Proposed structure for the remaining metal complexes |
where, M= Co or Cr (X= H2O, Y= Cl)
[Co(L)2 (H2O)(Cl)]+Cl-
[Cr(L)2 (H2O)(Cl)]2+2Cl-
M= Cu, Ni or Zn (X= H2O, Y= SO4) [ML2 (SO4) (H2O)].
3.9. Biological Activity
Most of the tested compounds have shown a promising biological activity against different types of Gram-positive and Gram-negative bacteria, and fungi. The bacteriostatic property of the metal complexes is higher than the ligand itself. The data are listed in Table 8 that show that E. coli was inhibited by all complexes. The importance of this lies in the fact that these complexes could reasonably be used for the treatment of some common diseases caused by E. coli, e.g., septicemia, gastroenteritis, urinary tract infections, and hospital-acquired infections. The mechanism of action of this complexes seems to be connected with the inhibition of phosphomannose isomerase, which is a key enzyme in the bio-synthesis of yeast cell walls [25Louie AY, Meade TJ. Metal complexes as enzyme inhibitors. Chem Rev 1999; 99(9): 2711-34.
[http://dx.doi.org/10.1021/cr9804285] [PMID: 11749498] ].
Mean zone of inhibition in mm ± Standard beyond well diameter (6mm) (100 ml was tested) produced on a range of clinically pathogenic microorganisms using (5mg per ml) concentration of tested samples. Results are depicted in Table 8.
CONCLUSION
In the present study new Schiff Base of (E)-N-(4-(thiophen-2-ylmethyleneamino) phenylsulfonyl) acetamide metal complexes were synthesized and then characterized by various physicochemical and spectral analytical techniques. The obtained complexes showed promising antimicrobial activity when compared to those of parent ligand.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
We are grateful to Eburon Organics (B-2310 Rijkevorsel, Belgium) for providing the authentic samples of CA and CAF, and to the Service Central d’Analyse (SCA), CNRS, F69360 Solaize, for recording the LC-MS spectra. We also thank the European SUDOE program for financial support. We would like to thank Saurabh Bansal, Eduardo Portillo Hahnefeld, and everyone who kindly volunteered on the spot to help us harvest our sea grass samples in various locations.
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publishers Website along with the published article.
REFERENCES
| [1] | Schiff H. Mittheilungen aus dem Universitätslaboratorium in Pisa: Eine neue Reihe organischer Basen. Justus Liebigs Ann Chem 1864; 131(1): 118-9. [http://dx.doi.org/10.1002/jlac.18641310113] |
| [2] | Popp FD, Kirsch W. Synthesis of potential anticancer agents V. schiff bases and related compounds1-2. J Org Chem 1961; 26(10): 3858-60. [http://dx.doi.org/10.1021/jo01068a056] |
| [3] | Hadjipavlou-Litina DJ, Geronikaki AA. Anti-inflammatory activity of some novel 1-[3-(aroylo)] and one 1-[3-(aryloxy)]-propyl aminothiazole in correlation with structure and lipophilicity. Arzneimittelforschung 1996; 46(8): 805-8. [PMID: 9125283] |
| [4] | Wadher SJ, Karande NA, Yeole PG. Synthesis and biological evaluation of schiff base of dapsone and their derivative as antimicrobial agents. Int J Pharm Tech Res 2009; 1(1): 12. |
| [5] | Cates LA, Rashed MS. Phosphorus GABA analogues as potential prodrugs. Pharm Res 1984; 1(6): 271-4. [http://dx.doi.org/10.1023/A:1016350119870] [PMID: 24277362] |
| [6] | Solak N, Rollas S. Synthesis and antituberculosis activity of 2-(aryl/alkylamino)-5-(4-aminophenyl)-1, 3, 4-thiadiazoles and their Schiff bases. ARKIVOC 2006; 12: 173-81. |
| [7] | Voronkov MG, Trukhina AA, Vlasova NN. Acyl iodides in organic synthesis: II. Reactions with acyclic and cyclic ethers. Russ J Org Chem 2002; 38(11): 1579-81. [http://dx.doi.org/10.1023/A:1022545630736] |
| [8] | Taggi AE, Hafez AM, Wack H, Young B, Ferraris D, Lectka T. The development of the first catalyzed reaction of ketenes and imines: catalytic, asymmetric synthesis of β-lactams. J Am Chem Soc 2002; 124(23): 6626-35. [http://dx.doi.org/10.1021/ja0258226] [PMID: 12047183] |
| [9] | Tsuge O, Kanemasa S. Recent advances in azomethine ylide chemistry InAdvances in heterocyclic chemistry Jan 1 Academic Press 1989; 45(23): 231-349. [http://dx.doi.org/10.1016/S0065-2725(08)60332-3] |
| [10] | Salameh AS, Tayim HA. Reaction of 2, 5-(Dibenzothiazolin-2-yl) thiophene with Some metal ions. Polyhedron 1983; 2(8): 829-34. [http://dx.doi.org/10.1016/S0277-5387(00)87213-7] |
| [11] | Tayim HA, Salameh AS. Reactions of 2-thiazolin-2-ylthiophene with some metal ions. Polyhedron 1983; 2(10): 1091-4. [http://dx.doi.org/10.1016/S0277-5387(00)81461-8] |
| [12] | Cartwright AJ, Jones P, Wolff JC, Evans EH. Detection of phosphorus tagged carboxylic acids using HPLC-SF-ICP-MS. J Anal At Spectrom 2005; 20(2): 75-80. [http://dx.doi.org/10.1039/b415962d] |
| [13] | Bauer AW, Kirby WM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol 1966; 45(4): 493-6. [http://dx.doi.org/10.1093/ajcp/45.4_ts.493] [PMID: 5325707] |
| [14] | Blasco FL, Perelló J, Latorre J. Borrás, S. Garciá-Granda, Cobalt(II), Nickel(II), and Copper(II) complexes of sulfanilamide derivatives: Synthesis, spectroscopic studies, and antibacterial activity. Crystal structure of. J Inorg Biochem 1996; 61(2): 143-54. [Co(sulfacetamide)2(NCS)2]. [http://dx.doi.org/10.1016/0162-0134(95)00053-4] [PMID: 8576708] |
| [15] | Agarwal PB, Kumar R, Aslam V, Khan A. Synthesis, physico-chemical and thermal characteristics of seven and ten coordinated complexes of trivalent lanthanides derived from 4[n-(cinnamalidene) amino] antipyrine semicarbazone and diphenyl sulfoxide. J Iran Chem Soc 2007; 4(1): 12. [http://dx.doi.org/10.1007/BF03245809] |
| [16] | Moustafa M. Spectrophotometric analysis, thermal analysis and gravimetric determination of some metal ions with oxime and Schiff’s base derivatives of N-furoylphenylhydroxylamine. J Therm Anal Calorim 1997; 50(3): 463-71. [http://dx.doi.org/10.1007/BF01980506] |
| [17] | Mohamed GG, Omar MM, Hindy AM. Metal complexes of Schiff bases: preparation, characterization, and biological activity. Turk J Chem 2006; 30(3): 361-82. |
| [18] | El-Gahami MA, Abdalla EM. Synthetic and spectroscopic studies of polymeric metal complexes involving 1, 4-[5-Disulphonyl-8-Hydroxyquinoline] piperazine. J Inorg Organomet Polym 2003; 13(1): 41-8. [http://dx.doi.org/10.1023/A:1022900914224] |
| [19] | Dobrzanska L, Wrzeszcz G, Grodzicki A, Rozploch F. Synthesis and properties of thiocyanato-bridged chromium (III)-copper (II) hydroxo complexes. Pol J Chem 2000; 74(7): 1017-22. |
| [20] | Janes R, Moore EA. Metal-ligand bonding 2004. |
| [21] | Gudasi KB, Patil MS, Vadavi RS, Shenoy RV, Patil SA, Nethaji M. X-ray crystal structure of the N-(2-hydroxy-1-naphthalidene) phenylglycine Schiff base. Synthesis and characterization of its transition metal complexes. Transition Met Chem 2006; 31(5): 580-5. [http://dx.doi.org/10.1007/s11243-006-0031-3] |
| [22] | Chen W, Li Y, Cui Y, Zhang X, Zhu HL, Zeng Q. Synthesis, molecular docking and biological evaluation of Schiff base transition metal complexes as potential urease inhibitors. Eur J Med Chem 2010; 45(10): 4473-8. [http://dx.doi.org/10.1016/j.ejmech.2010.07.007] [PMID: 20691510] |
| [23] | Creaven BS, Duff B, Egan DA, et al. Anticancer and antifungal activity of copper (II) complexes of quinolin-2 (1H)-one-derived Schiff bases. Inorg Chim Acta 2010; 363(14): 4048-58. [http://dx.doi.org/10.1016/j.ica.2010.08.009] |
| [24] | Raman N, Jeyamurugan R, Senthilkumar R, Rajkapoor B, Franzblau SG. In vivo and in vitro evaluation of highly specific thiolate carrier group copper(II) and zinc(II) complexes on Ehrlich ascites carcinoma tumor model. Eur J Med Chem 2010; 45(11): 5438-51. [http://dx.doi.org/10.1016/j.ejmech.2010.09.004] [PMID: 20864225] |
| [25] | Louie AY, Meade TJ. Metal complexes as enzyme inhibitors. Chem Rev 1999; 99(9): 2711-34. [http://dx.doi.org/10.1021/cr9804285] [PMID: 11749498] |