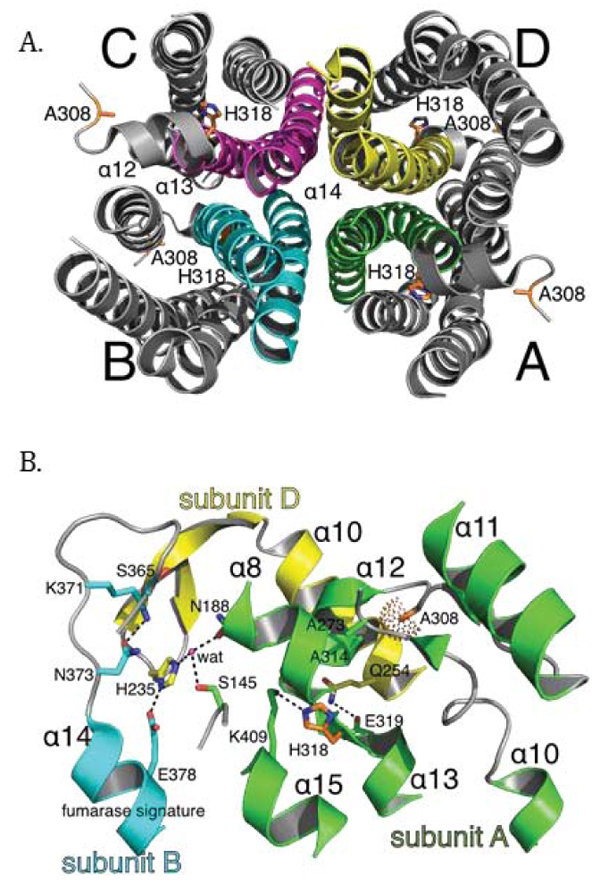
Fig. (7)
The putative structural impact of the A308T and H318Y variants at the multi-subunit active site in the human fumarase structure (PDB ID: 3E04 [40]). (A) The human fumarase tetramerization interface has been displayed looking down related D2 domains. For clarity, D1 and D3 have been removed from this image. Upon oligomerization, α-helix 14 lies at the center of the homotetramer. Additionally, α-helices 12 and 13 contribute residues to the tetramer interface. (B) The three-subunit fumarase active site has been depicted with emphasis upon the locations of A308 and H318 in context to the A-D dimer interface and fumarase signature sequence (GS365SxxPxK371xN373). Dashed lines depict hydrogen bonds and the dot surface reflects the A308 Cβ atom van der Waal radius. Residual monomer elements have been colored as depicted in Fig. (1B). The side chain atoms for A308 and H318 have been depicted in stick format and colored by atom type (red-oxygen, blue-nitrogen, orange-carbon). The fumarase active site water (wat) has been depicted as a magenta colored sphere.