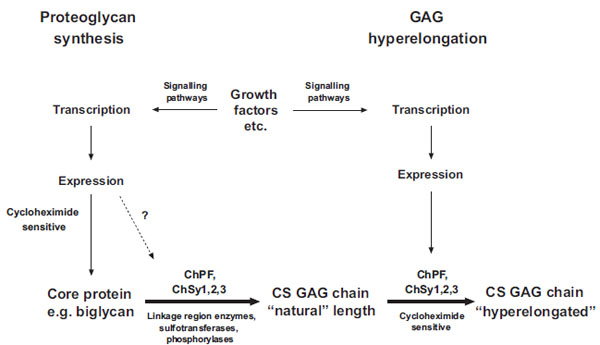
Fig. (2) Schema of the biochemical processes leading to the synthesis of natural chondroitin sulfate glycosaminoglycan (GAG) chains and the hyperelongation of GAG chains elicited by multiple cell signaling pathways resulting from the action of growth factor such as PDGF and TGFb. ChSy chondroitin synthase; ChPF chondroitin polymerizing factor. The scheme to the left shows the normal biosynthesis of a pro-teoglycan core protein which is new protein and therefore cycloheximide sensitive but is also subject, based on individual and specific core proteins, to up regulation by growth factors. Various combinations, at least pairs of polymerases (see Table 1) add monosaccharides to the preformed tetrasaccharide linkage region on a serine residue on the core protein to produce the “natural” length glycosaminoglycan (GAG) chain. To the right of the scheme it is indicated that growth factors can intervene to result in the production of longer, so called hyperelon-gated GAG chains and although this is cycloheximide sensitive (see text) and acutely requires new protein synthesis the site at which the new protein synthesis occurs, either in the signaling pathway or in the actual transporters and enzymes that synthesis GAGs is unknown. The GAG elongation in response to growth factors is of the order of 20 per cent but this is sufficient to half the affinity of binding of the GAG chain to LDL.