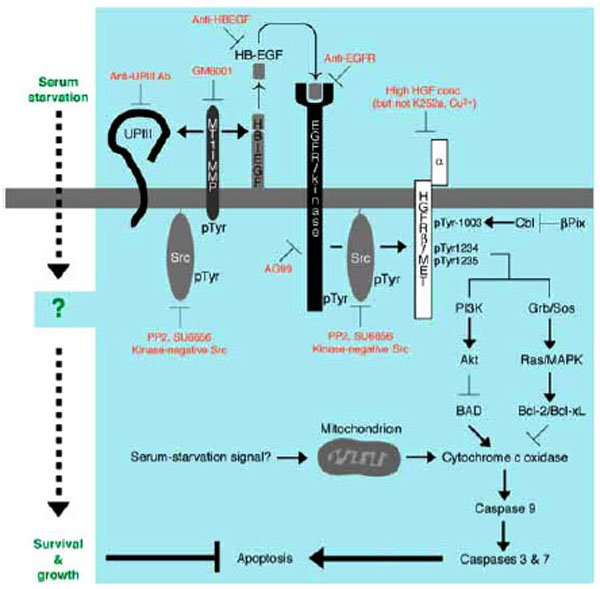
Fig. (4) Anti-apoptotic mechanism of survival and growth of serum-starved 5637 human bladder carcinoma cells. Serum starvation signal may trigger both non-genomic (e.g. catalytic and/or physical protein interactions) and genomic responses (gene expression and/or protein synthesis) in 5637 cells. At early phase of signal transduction, activation of Src by unknown mechanism, activation of MT1-MMP by tyro-sine phosphorylation and/or increased protein expression, secretion of soluble HB-EGF into the culture medium, and proteolysis of uroplakin III are operating. Secreted HB-EGF binds to and activates EGFR/kinase. Src may also be activated under the control of EGFR. Src and EGFR phosphorylate β-subunit of HGFR/c-Met (p145) on tyrosine residues 1003, 1234, and 1235. These phosphotyrosine residues in β-subunit of HGFR/c-Met may be responsible for up-regulating anti-apoptotic ability of cells through PI 3-kinase-Akt pathway and/or Grb2-Sos-Ras-MAPK pathway: the former contributes to phosphorylation and inactivation of BAD, a pro-apoptotic component of Bcl-2 family, and the latter contributes to induction of Bcl-2 and Bcl-xL, both of which are anti-apoptotic components of Bcl-2 family. These components act positively or negatively on cytochrome c oxidase, which will be released from mitochondria upon mitochondria-mediated apoptosis in response to metabolically sensed death signals (i.e. serum starvation). Cytochrome c oxidase, if released, promotes sequential activation of caspase 9 and caspase 3/7, latter of which directly contributes to apoptotic cellular processes. Thus, serum-independent survival and growth of 5637 cells may involve suppression of pro-apoptotic pathway and/or activation of anti-apoptotic pathway