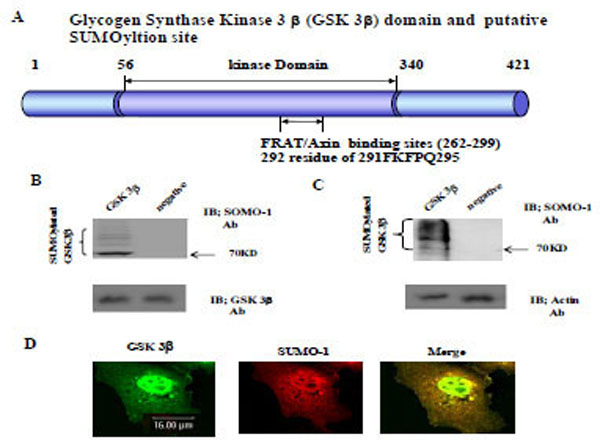
Fig. (1). GSK 3β functional domain and SUMOylation The Glycogen synthase kinase 3β (GSK 3β) functional domains (its protein kinase and FRAT/Axin binding domain) and the putative SU-MOylation site (K292 in 291FKFPQ295) is indicated (A). GSK 3β SUMO mutant (K292R) was constructed by site directed mutagenesis. GSK 3β SUMO mutant (K292R) was inserted into GST fusion (for bacteria) and Ha fusion (for cell line) expression vectors. (B) GSK 3β wild type (wt) protein that was purified from E. coli was incubated with a SUMOylation assay kit (See Material and method). For the negative control, the same assay conditions were used without ATP (right lane). A western blot of the same sample was performed with GSK 3β monoclonal antibody to monitor the protein amount in the experiment (at bottom). SUMOylated GSK 3β, as several high molecular weight protein bands, was indicated. (C) A western bolt of the immunopurified GSK 3β from COS-1 was performed using the SUMO-1 specific antibody. SUMOylation of GSK 3β was detected as high molecular weight protein bands, as indicated (left lane). For the negative control, an unrelated mouse antibody was used (right lane). To monitor the total protein amount to be used in the cell lysates, the western blot was per-formed with actin monoclonal antibody (bottom). (D) Confocal microscopic analysis of endogenous GSK 3β wt (green color) and SUMO-1 (red color). GSK 3β was detected in both the cytoplasm and nuclear region. The SUMO-1 modification proteins were mainly detected in the nuclear region (yellow color). All the figures in this article represent results from three experiments repeated independently.