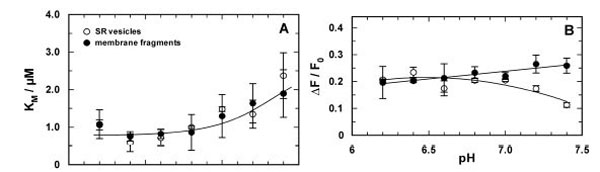
Fig. (5) Comparison of the pH-dependent Ca2+ binding in the E2P conformation of the SR Ca-ATPase in which the ion-binding sites are accessible from the luminal side of the membrane. Titration experiments were analyzed and the characteristic fitting parameters in the Hill function, KM and &ΔF/F0, were plotted against the pH of the buffer solution in the cuvette. (A) The half-saturating Ca2+ concentration, KM, from both preparation did not significantly differ from each other over the pH range covered by experiments.Below pH 7 it was approximately constant with a value of 0.86±0.06 µM and increased to about 2 µM at pH 7.4. In contrast to the results in the E1 conformation no competition between Ca2+ and H+ is evident. (B) At pH 7 and below the fluorescence change upon addition of saturating Ca2+ (here plotted as absolute value) is the same for the experiments with both preparations. The decrease of ΔF/F0 in the case of the SR vesicles may not be significant, it was not found before [21]