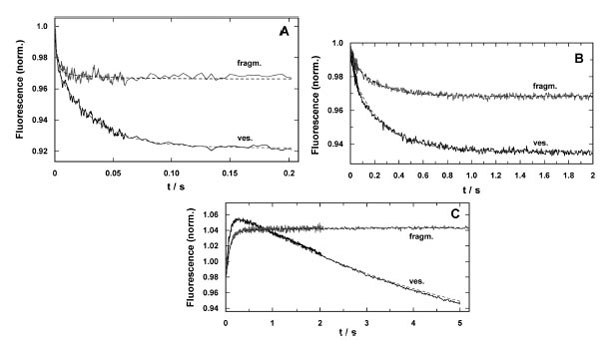
Fig. (6) Time-resolved response of the fluorescence signal in concentration-jump experiments performed by UV-flash induced substrate release from a caged precursor with SR vesicles (ves.) and purified membrane fragments (fragm.). (A) pH jump ex-periment in the electrolyte that maintains the SR Ca-ATPase in its E1 conformation. The release of protons causes a right shift in the reaction sequence, E1 ↔ H2E1 ↔ H4E1 [5]. In both preparations the time course could be fitted with a sum of two expo-nential functions (Eq. 2). While the time constants were comparable, the amplitudes differed significantly, F1/F2(ves.) = 0.5 and F1/F2(fragm.) = 5. (B) Ca2+-concentration jump in the E1 conformation of the SR Ca-ATPase lead to a right shift in the reaction sequence, E1 ↔ CaE1 ↔ Ca2E1. Again, the fits of the data with Eq. (2) revealed comparable time constants, the ampli-tude ratios, F1/F2(ves.) = 0.28 and F1/F2(fragm.) = 0.21, were not to far from each other, however, the total fluorescence ampli-tude differed by more than a factor of 2. (C) ATP-jump experiments were performed under the condition that release of the nucleotide triggered the reaction, Ca2E1 → (Ca2)E1-P → P-E2(Ca2) → P-E2, and then all substrates are present to allow pump turnover, controlled by the rate-limiting step, P-E2 → P-E2H2 [22]. While the SR vesicles show a biphasic behavior the mem-brane fragments exhibit only the rising phase of the fluorescence with a time constant similar to that for the Ca-ATPase in the vesicles