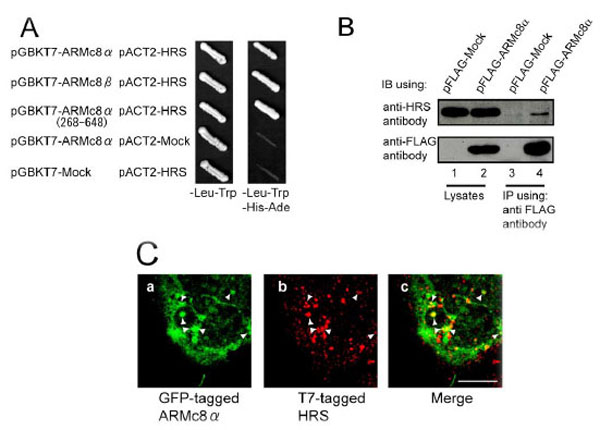
Fig. (1). HRS associates with ARMc8α in vivo, and exogenously expressed ARMc8α co-localizes with exogenously expressed T7-tagged HRS in HEK293 cells A. AH109 yeast strains were transformed with bait constructs (pGBKT7-ARMc8α, pGBKT7-ARMc8β, pGBKT7-ARMc8α (amino acids 268–648) or pGBKT7-Mock) and prey constructs (pACT2-HRS or pACT2-Mock), and the transformants were inoculated onto a plate containing SD medium lacking leucine and tryptophan to select positive clones transformed with both constructs (bait and prey) (left panel, - Leu-Trp). Colonies positive for growth were restreaked onto a plate of SD medium lacking adenine, histidine, leucine, and tryptophan to select positive clones expressing the reporter genes ADE and HIS3 (right panel, -Ade-His-Leu-Trp).B. CHO cells were transfected with pFLAG-ARMc8α or pFLAG-Mock, and the cell extracts were immunoprecipitated with an anti-FLAG antibody. The lysates and immunoprecipitates were analyzed by SDS-PAGE, followed by immunoblotting with an anti-FLAG antibody or an anti-HRS antibody. IB, immunoblot. IP, immunoprecipitation.C. HEK293 cells were co-transfected with pEGFP-ARMc8α and pT7-HRS. Cells were immunostained with a T7•tag antibody and analyzed by confocal microscopy, as described in Material and Methods. GFP-tagged ARMc8α was distributed within the cytoplasm (panel a), as was T7-tagged HRS (panel b). Panel c is a merged image of the two exogenous proteins. Arrowheads indicate typical co-localization. Scale bar represents 10 µm.