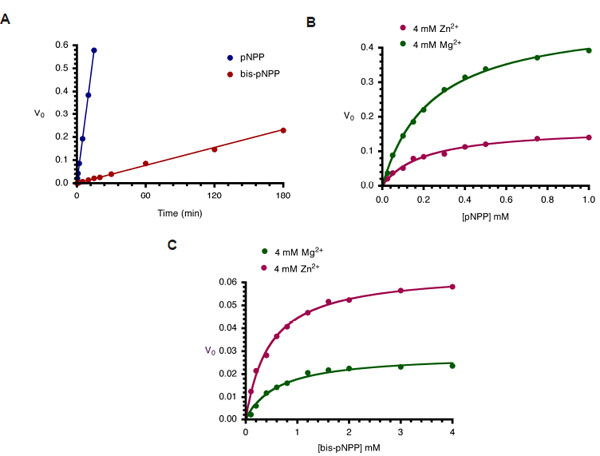
Fig. (3) Kinetics of monoesterase and diesterase activities of CIAP. (A). Time course of hydrolysis of pNPP and bis-pNPP. Reaction
mixtures (200 µl) containing 25 mM Tris-HCl (pH 8.5), 2.5 mM p-nitrophenyl phosphate (or bis-p-nitrophenyl phosphate), 100 µM ZnCl2, 1
mM MgCl2, and 10 µM CIAP were incubated at 37 °C for the indicated time. Reactions were initiated by the addition of the substrate to the
reaction mixture and stopped by the addition of 1.0 ml 0.5 M NaOH. Activities are expressed as change in A410 per minute for
phosphomonoesterase activity and change in A410 per hour for the phosphodiesterase activity. (B) Effects of Zn2+ and Mg2+ on kinetics of
CIAP-catalyzed hydrolysis of pNPP. The initial rates of hydrolysis of increasing concentrations of pNPP by 10 µM CIAP at 37 °C in the
presence of 4 mM Zn2+ or 4 mM Mg2+ were determined. The data were fitted to the Michaelis-Menten curve using GraphPad Prism curvefitting
software. The concentrations of pNPP used were 0.025, 0.05, 0.1, 0.15, 0.2, 0.3, 0.4, 0.5, 0.75, and 1.0 mM. (C) Effects of Zn2+ and
Mg2+ on kinetics of CIAP-catalyzed hydrolysis of bis-pNPP. Reactions and data analysis were carried out as described in (B). The
concentrations of bis-pNPP used were 0.1, 0.2, 0.4, 0.6, 0.8, 1.2, 1.6, 2.0, 3.0, and 4.0 mM.