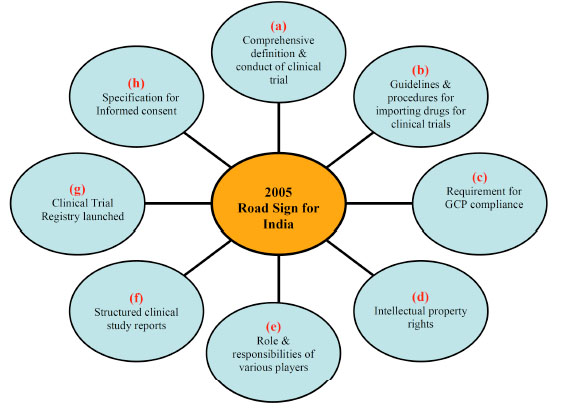
Fig. (1) Broad outline of changes takes place favouring clinical research in India in 2005. (a) Govt. of India meticulously defines the various
terminology & way to conduct the clinical trials. (b) Specification for drug import in India. (c) more focus on GCP compliance by direct or
indirect participant in clinical trials. (d) protection of intellectual property of individual or of an organization. (e) defining the role &
responsibilities of sponsor, investigator, ethics committee & others. (f) well structure & formatting of clinical study report. (g) to register a
clinical trial before testing on animal or human being. (h) protection of human being by well specified informed consent documents.