- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

The Open Fuels & Energy Science Journal
(Discontinued)
ISSN: 1876-973X ― Volume 11, 2018
Effect of CaO and Fe2O3 on Partitioning of As and S within Ash Fractions from Fluidised-Bed Co-Combustion of Coal and Wastes
Lucie Bartoňová*
Abstract
Possible interaction of volatilized As and S with CaO and Fe2O3 (creating solid product) could efficiently improve coal combustion flue gas cleaning. For this reason, S-CaO, As-CaO, S- Fe2O3 and As- Fe2O3 relationships were evaluated in bottom ash and fly ash fractions from fluidised-bed co-combustion of coal and wastes (and limestone as desulphurization additive) through calculation of correlation coefficients and composition of magnetic concentrates. It was concluded that S exhibited a dominant association with CaO while As exhibited affinity to both CaO and Fe2O3 - the significance differed a little in bottom ash and fly ash. In the bottom ash, the affinity of As to CaO was more significant, while in the fly ash the association to Fe2O3 slightly prevailed.
Article Information
Identifiers and Pagination:
Year: 2018Volume: 11
First Page: 81
Last Page: 90
Publisher Id: TOEFJ-11-81
DOI: 10.2174/1876973X01811010081
Article History:
Received Date: 21/3/2018Revision Received Date: 15/05/2018
Acceptance Date: 12/06/2018
Electronic publication date: 31/7/2018
Collection year: 2018
open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
* Address correspondence to this author at the Regional Materials Science and Technology Centre, VŠB-Technical University of Ostrava, Tř. 17. listopadu 15, Ostrava – Poruba, Czech Republic, Tel: +420-59 699 1514; E-mail: lucie.bartonova@vsb.cz
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 21-3-2018 |
Original Manuscript | Effect of CaO and Fe2O3 on Partitioning of As and S within Ash Fractions from Fluidised-Bed Co-Combustion of Coal and Wastes | |
1. INTRODUCTION
Fluidised bed combustion provides numerous advantages in comparison with predominant combustion systems, such as high combustion efficiency, reduced coal crushing, ability to use low-grade coals and alternative fuels (wastes, biomass, sewage sludge) [1Raclavská, H.; Juchelková, D.V.; Roubíček, V.; Matysek, D. Energy utilization of biowaste-sunflower seed hulls for co-firing with coal. Fuel Process. Technol., 2011, 92, 13-20.
[http://dx.doi.org/10.1016/j.fuproc.2010.03.006] , 2Raclavská, H.; Juchelková, D.; Škrobánková, H.; Wiltowski, T.; Campen, A. Conditions for energy generation as an alternative approach to compost utilization. Environ. Technol., 2011, 32(3-4), 407-417.
[http://dx.doi.org/10.1080/09593330.2010.501089] [PMID: 21780708] ], etc. One of the most important advantages is low combustion temperature (below ash fusion temperature) facilitating easier ash removal and significantly reducing air pollution. Moreover, due to ground limestone/dolomite / CaO added during the combustion, the efficient desulphurization of emissions could be achieved.
The total amount of emissions from coal combustion power station is a complex phenomenon and depends on both operating conditions and the characteristics of the coal (e.g. trace elements in the coal) [3Wagner, N.J.; Hlatshwayo, B. The occurrence of potentially hazardous trace elements in five Highveld coals, South Africa. Int. J. Coal Geol., 2005, 63, 228-246.
[http://dx.doi.org/10.1016/j.coal.2005.02.014] , 4Wagner, N.J.; Tlotleng, M.T. Distribution of selected trace elements in density fractionated Waterberg coals from South Africa. Int. J. Coal Geol., 2012, 94, 225-237.
[http://dx.doi.org/10.1016/j.coal.2012.01.005] ]. Despite the progress in flue-gas cleaning technologies, fluidised bed combustion is still accompanied by emissions of numerous pollutants, such as heavy metals, toxic trace elements, or organic compounds etc. [5Klika, Z.; Bartoňová, L.; Spears, D.A. Effect of boiler output on trace element partitioning during coal combustion in two fluidised-bed power stations. Fuel, 2001, 80, 907-917.
[http://dx.doi.org/10.1016/S0016-2361(00)00164-2] , 6Wagner, N.J. The characterization of weathered discard coals and their behaviour during combustion. Fuel, 2008, 87, 1687-1697.
[http://dx.doi.org/10.1016/j.fuel.2007.09.009] ]. As the retention efficiency of fabric filters and electrostatic precipitators is relatively high, the capture of gaseous fractions of volatile elements, such as Hg, As, Cl, Br, etc [7Germani, M.S.; Zoller, W.H. Vapor-phase concentrations of arsenic, selenium, bromine, iodine, and mercury in the stack of a coal-fired power plant. Environ. Sci. Technol., 1988, 22(9), 1079-1085.
[http://dx.doi.org/10.1021/es00174a013] [PMID: 22148663] , 8Meij, R.; Janssen, L.H.J.M.; Van Der Kooij, J. Air pollutant emissions from coal-fired power stations. Kema Scientific & Technical Reports, 1986, 4, 51-69.] is more problematic (volatilized elements in gaseous form can easily pass through the particulate control devise).
To solve this problem, there is an effort to capture the volatile elements from flue gas onto solid adsorbent. Activated carbon is one of the most effective adsorbents for the retention of Hg [9Scala, F.; Chirone, R.; Lancia, A. Elemental mercury vapor capture by powdered activated carbon in a fluidised bed reactor. Fuel, 2011, 90, 2077-2082.
[http://dx.doi.org/10.1016/j.fuel.2011.02.042] , 10Vidic, R.D.; McLaughlin, J.B. Uptake of elemental mercury vapors by activated carbons. J. Air Waste Manag. Assoc., 1996, 46(3), 241-250.
[http://dx.doi.org/10.1080/10473289.1996.10467458] [PMID: 28065140] ] and it was tested for the retention of As and Se [11López-Antón, M.A.; Díaz-Somoano, M.; Fierro, J.L.G.; Martínez-Tarazona, M.R. Retention of arsenic and selenium compounds present in coal combustion and gasification flue gases using activated carbons. Fuel Process. Technol., 2007, 88, 799-805.
[http://dx.doi.org/10.1016/j.fuproc.2007.03.005] ] as well. However, to reduce the cost, cheaper alternatives are also being sought. Unburned carbon (in fly ash) is one of the most promising alternatives [12Hower, J.; Thomas, G.A.; Clifford, D.S.; Eady, J.D. Petrography and Chemistry of High-Carbon Fly Ash form the Shawnee Power Station, Kentucky. Energy Sources, 1996, 18, 107118.
[http://dx.doi.org/10.1080/00908319608908751] -14Raclavská, H.; Hlavsová, A.; Juchelková, D.; Zajonc, O. The geochemical properties of carbon black from pyrolysis of municipal waste. Inzynieria Mineralna, 2013, 1, 19-27.] and was studied in terms of possible flue gas cleaning from Hg [15Hower, J.C.; Maroto-Valer, M.; Taulbee, D.N.; Sakulpitakphon, T. Mercury capture by distinct fly ash carbon forms. Energy Fuels, 2000, 14, 224-226.
[http://dx.doi.org/10.1021/ef990192n] -17Hower, J.C.; Senior, C.L.; Suuberg, E.M.; Hurt, R.H.; Wilcox, J.L.; Olson, E.S. Mercury capture by native fly ash carbons in coal-fired power plants. Pror. Energy Combust. Sci., 2010, 36(4), 510-529.
[http://dx.doi.org/10.1016/j.pecs.2009.12.003] [PMID: 24223466] ] and some other trace elements [18Bartoňová, L. Unburned carbon from coal combustion: An overview. Fuel Process. Technol., 2015, 134, 136-158.
[http://dx.doi.org/10.1016/j.fuproc.2015.01.028] -21Bartoňová, L.; Klika, Z.; Spears, D.A. Characterization of unburned carbon from ash after bituminous coal and lignite combustion in CFBs. Fuel, 2007, 86, 455-463.
[http://dx.doi.org/10.1016/j.fuel.2006.07.024] ]. The major advantage of unburned carbon is its porosity and higher specific surface area [18Bartoňová, L. Unburned carbon from coal combustion: An overview. Fuel Process. Technol., 2015, 134, 136-158.
[http://dx.doi.org/10.1016/j.fuproc.2015.01.028] , 22Hower, J.C.; Groppo, J.G.; Graham, U.M.; Ward, C.R.; Kostova, I.J.; Maroto-Valer, M.M.; Dai, S. Coalderived unburned carbons in fly ash: A review. Int. J. Coal Geol., 2017, 179, 11-27.
[http://dx.doi.org/10.1016/j.coal.2017.05.007] ] in comparison with other ash grains; in combination with some functional groups it improves the retention of inorganic species [15Hower, J.C.; Maroto-Valer, M.; Taulbee, D.N.; Sakulpitakphon, T. Mercury capture by distinct fly ash carbon forms. Energy Fuels, 2000, 14, 224-226.
[http://dx.doi.org/10.1021/ef990192n] -17Hower, J.C.; Senior, C.L.; Suuberg, E.M.; Hurt, R.H.; Wilcox, J.L.; Olson, E.S. Mercury capture by native fly ash carbons in coal-fired power plants. Pror. Energy Combust. Sci., 2010, 36(4), 510-529.
[http://dx.doi.org/10.1016/j.pecs.2009.12.003] [PMID: 24223466] ] from flue gas or can be used for the retention of dyes from aqueous solutions [23Bartoňová, L.; Ruppenthalová, L.; Ritz, M. Adsorption of Naphthol Green B on unburned carbon: 2- and 3 parameter linear and non-linear equilibrium modelling. Chin. J. Chem. Eng., 2017, 25, 37-44.
[http://dx.doi.org/10.1016/j.cjche.2016.05.035] ]. In addition to carbonaceous materials, the retention of volatile elements was tested on kaolinite [24Wendt, J.O.L.; Lee, S.J. High-temperature sorbents for Hg, Cd, Pb and other trace metals: Mechanisms and applications. Fuel, 2010, 89, 894-903.
[http://dx.doi.org/10.1016/j.fuel.2009.01.028] ], MnOx [25Scala, F.; Anacleria, C.; Cimino, S. Characterization of a regenerable sorbent for high temperature elemental mercury capture from flue gas. Fuel, 2013, 108, 13-18.
[http://dx.doi.org/10.1016/j.fuel.2010.12.028] , 26Scala, F.; Cimino, S. Elemental mercury capture and oxidation by a regenerable manganese-based sorbent: The effect of gas composition. Chem. Eng. J., 2015, 278, 134-139.
[http://dx.doi.org/10.1016/j.cej.2014.11.094] ] or desulphurization additives / calcareous adsorbents [27Scala, F.; Chirone, R.; Meloni, P.; Carcangiu, G.; Manca, M.; Mulas, G.; Mulas, A. Fluidised bed desulphurization using lime obtained after slow calcination of limestone particles. Fuel, 2013, 114, 99105.
[http://dx.doi.org/10.1016/j.fuel.2012.11.072] -29Bartoňová, L.; Klika, Z. Effect of CaO on retention of S, Cl, Br, As, Mn, V, Cr, Ni, Cu, Zn, W and Pb in bottom ashes from fluidized-bed coal combustion power station. J. Environ. Sci. (China), 2014, 26(7), 1429-1436.
[http://dx.doi.org/10.1016/j.jes.2014.05.008] [PMID: 25079991] ]. It is worth mentioning in this context that the percentage of CaO in fluidised-bed ashes is typically higher than that of unburned carbon (in some fractions it can reach even 50%) [29Bartoňová, L.; Klika, Z. Effect of CaO on retention of S, Cl, Br, As, Mn, V, Cr, Ni, Cu, Zn, W and Pb in bottom ashes from fluidized-bed coal combustion power station. J. Environ. Sci. (China), 2014, 26(7), 1429-1436.
[http://dx.doi.org/10.1016/j.jes.2014.05.008] [PMID: 25079991] ].
Desulphurization additives (lime, limestone etc.) are currently used for the retention of S. Except S, promising results on the possible retention of As on Ca-bearing compounds were reported as well [11López-Antón, M.A.; Díaz-Somoano, M.; Fierro, J.L.G.; Martínez-Tarazona, M.R. Retention of arsenic and selenium compounds present in coal combustion and gasification flue gases using activated carbons. Fuel Process. Technol., 2007, 88, 799-805.
[http://dx.doi.org/10.1016/j.fuproc.2007.03.005] , 29Bartoňová, L.; Klika, Z. Effect of CaO on retention of S, Cl, Br, As, Mn, V, Cr, Ni, Cu, Zn, W and Pb in bottom ashes from fluidized-bed coal combustion power station. J. Environ. Sci. (China), 2014, 26(7), 1429-1436.
[http://dx.doi.org/10.1016/j.jes.2014.05.008] [PMID: 25079991] , 30Clemens, A.H.; Damiano, L.F.; Gong, D.; Metheson, T.W. Partitioning behaviour of some toxic volatile elements during stoker and fluidised-bed combustion of alkaline sub-bituminous coal. Fuel, 1999, 78, 1379-1385.
[http://dx.doi.org/10.1016/S0016-2361(99)00066-6] ]. However, some recent studies concluded that not only Ca but also Fe could provide sites for As association [11López-Antón, M.A.; Díaz-Somoano, M.; Fierro, J.L.G.; Martínez-Tarazona, M.R. Retention of arsenic and selenium compounds present in coal combustion and gasification flue gases using activated carbons. Fuel Process. Technol., 2007, 88, 799-805.
[http://dx.doi.org/10.1016/j.fuproc.2007.03.005] , 31Seames, W.S.; Wendt, J.O.L. Regimes of association of arsenic and selenium during pulverized coal combustion. Proc. Combust. Inst., 2007, 31, 2839-2846.
[http://dx.doi.org/10.1016/j.proci.2006.08.066] -33Low, F.; Zhang, L. Arsenic emissions and speciation in the oxy-fuel fly ash collected from lab-scale droptube furnace. Proc. Combust. Inst., 2013, 34, 2877-2884.
[http://dx.doi.org/10.1016/j.proci.2012.05.026] ]. The conclusion that As can be captured from flue gas on active sites of both Ca and Fe was based on thermodynamic equilibrium calculations [32Contreras, M.L.; Arostegui, J.M.; Armesto, L. Arsenic interactions during co-combustion processes based on thermodynamic equilibrium calculations. Fuel, 2009, 88, 539-546.
[http://dx.doi.org/10.1016/j.fuel.2008.09.028] ] and laboratory-scale combustion [11López-Antón, M.A.; Díaz-Somoano, M.; Fierro, J.L.G.; Martínez-Tarazona, M.R. Retention of arsenic and selenium compounds present in coal combustion and gasification flue gases using activated carbons. Fuel Process. Technol., 2007, 88, 799-805.
[http://dx.doi.org/10.1016/j.fuproc.2007.03.005] , 31Seames, W.S.; Wendt, J.O.L. Regimes of association of arsenic and selenium during pulverized coal combustion. Proc. Combust. Inst., 2007, 31, 2839-2846.
[http://dx.doi.org/10.1016/j.proci.2006.08.066] , 33Low, F.; Zhang, L. Arsenic emissions and speciation in the oxy-fuel fly ash collected from lab-scale droptube furnace. Proc. Combust. Inst., 2013, 34, 2877-2884.
[http://dx.doi.org/10.1016/j.proci.2012.05.026] ]. Since As retention is a complex phenomenon, the results from real power stations are needed. Besides, fluidised-bed coal combustion ashes are advantageous for this study due to naturally high levels of Ca-bearing minerals (desulphurization additives) that can affect Fe2O3 interaction.
Possible association of volatilized As and S with CaO and Fe2O3 (creating solid product) could efficiently improve coal combustion flue gas cleaning. For this reason, herein, the relationships between S and As with CaO and Fe2O3 were evaluated in bottom ash and fly ash fractions from fluidised-bed co-combustion of coal and wastes (and limestone as desulphurization additive) through calculation of correlation coefficients and composition of magnetic concentrates.
2. METHODS
The samples of Bottom Ash (BA) and Fly Ash (FA) were collected at atmospheric circulating fluidisedbed power station where lignite was co-combusted with wood, bark, sewage sludge and solid recovered fuel (blend of shredded paper, plastics, wood, textiles, carpets etc.). Desulphurization of emissions was achieved by adding limestone as (dry) desulphurization additive. Fluidised-bed combustion typically does not require powdered coal (combustion efficiency is achieved by circulating in fluidised-bed and longer dwell time); therefore, only limestone was finely ground for efficient interaction with S in flue gas. Efficient combustion can be documented by low unburned carbon content in both BA (< 3%) and FA (< 1%).
During the whole combustion test, fractional samples of bottom ash and fly ash were collected at regular time intervals and mixed properly. From these composite samples, representative subsamples were separated by quartering for further preparation and analyses in the laboratory.
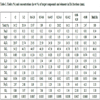
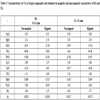
From bulk BA and FA samples, the following particle size fractions were separated by sieving. BA fractions: >2 mm, 1-2 mm, 0.6-1.0 mm, 0.5-0.6 mm, 0.4-0.5 mm, 0.2-0.4 mm, 0.1-0.2 mm, 0.08-0.1 mm and <0.08 mm; FA fractions: >0.1 mm, 0.09-0.1 mm, 0.08-0.09 mm, 0.071-0.08 mm, 0.063-0.071 mm, 0.056-0.063 mm, 0.053-0.056 mm, 0.050-0.053 mm, 0.045-0.050 mm, 0.036-0.045 mm, 0.032-0.036 mm and <0.032 mm (percentage yields are given in Tables 1 and 2.
In order to distinguish between CaO and Fe2O3 associations, non-magnetic / magnetic fractions of BA and FA were analyzed for target compounds and elements. Due to quite different granulometry of BA (coarse grains) and FA (fine particles), similar particle-size fractions of BA (0.1-0.2 mm) and FA (> 0.1 mm) were used for the magnetic separation. Magnetic concentrates were manually separated (when dry) using a magnet.
The samples of BA and FA were milled using vibration mill Retsch MM400 and (using binding additive Boreox) 40 mm pellets were pressed on Vaneox automatic press. The elemental analysis was conducted on wavelength-disperzive X-ray fluorescence spectrometer ARL PERFORM´X 4200 W (Switzerland) equipped with 2 detectors, 9 crystals and 4 collimators.
3. RESULTS AND DISCUSSION
3.1. Concentrations of Elements in Ash Fractions
Chemical analysis of particle-size fractions of BA is given in Table 1 and the same data for FA are listed in Table 2. Unlike FA, elemental concentrations in BA fractions exhibit a wide range: CaO and SiO2 concentrations vary from 2 to 46% and from 15 to 54%, respectively. The considerable differences in the coarser and finer fractions were brought about by adding limestone as a desulphurization additive. For the effective retention of S, limestone was finely ground while SiO2 (and some aluminosilicates) originated from relatively larger grains. Therefore, finer fractions are highly enriched in CaO while coarse fractions contain much more SiO2. Unlike BA, the CaO and SiO2 concentrations in FA vary “only“ from 10.72 to 14.23% and from 39.65 to 44.53%, respectively.
For easier evaluation, the distribution trends of As and S are depicted in Figs. (1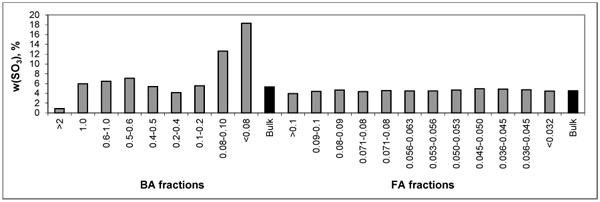 and 2
and 2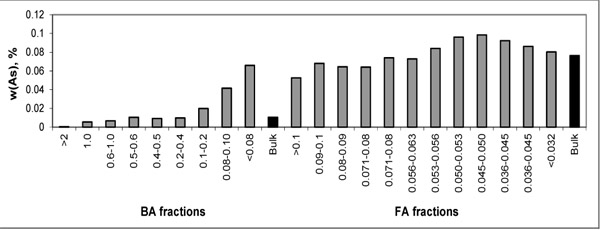 ) showing higher concentrations of As in FA fractions and some increase in finer fractions of BA. Such behaviour corresponds with volatile character of As. The highest S concentrations are present in the finer BA fractions.
) showing higher concentrations of As in FA fractions and some increase in finer fractions of BA. Such behaviour corresponds with volatile character of As. The highest S concentrations are present in the finer BA fractions.
 |
Fig. (1) Distribution of SO3 within particle-size fractions of BA and FA. |
 |
Fig. (2) Distribution of As within particle-size fractions of BA and FA. |
3.2. Relationships Between Concentrations of Target Elements
Partitioning of As and S within particle size fractions was evaluated in terms of distribution of the major element
oxides, namely CaO and Fe2O3.
3.2.1. Sulphur
Relationships between concentrations of SO3 and those of CaO and Fe2O3 are depicted in Fig. (3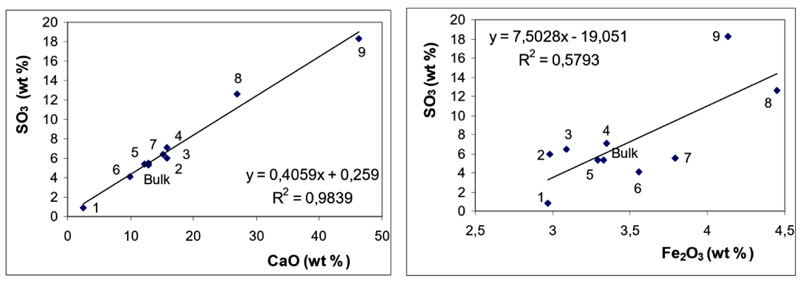 ) for BA and in Fig. (4
) for BA and in Fig. (4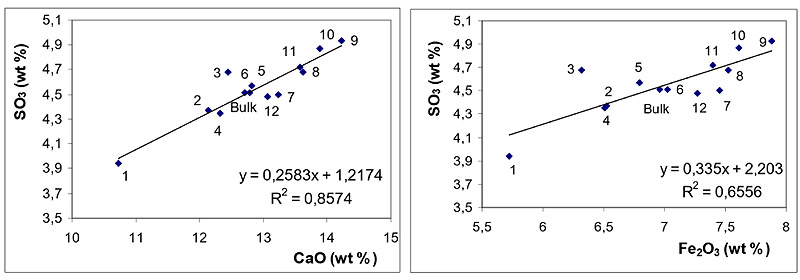 ) for FA fractions.
) for FA fractions.
Figs. (3 and 4
and 4 ) clearly document that SO3 is predominantly associated with CaO both in BA and in FA fractions. The effect of Fe2O3 on SO3 distribution is of less significance in these samples.
) clearly document that SO3 is predominantly associated with CaO both in BA and in FA fractions. The effect of Fe2O3 on SO3 distribution is of less significance in these samples.
Association of SO3 with CaO documents the efficient retention of sulphur at coal combustion, which is usually explained through the reactions see equation (1) and (2) [30Clemens, A.H.; Damiano, L.F.; Gong, D.; Metheson, T.W. Partitioning behaviour of some toxic volatile elements during stoker and fluidised-bed combustion of alkaline sub-bituminous coal. Fuel, 1999, 78, 1379-1385.
[http://dx.doi.org/10.1016/S0016-2361(99)00066-6] ]:
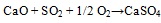 |
(1) |
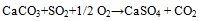 |
(2) |
3.2.2. Arsenic
Mutual relationships (correlations) between the concentrations of As and those of CaO and Fe2O3 are shown in Fig. (5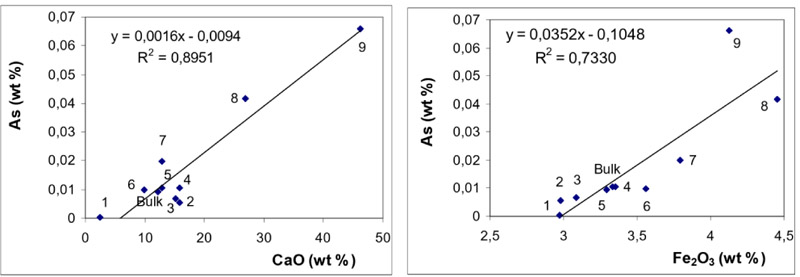 ) for BA and in Fig. (6
) for BA and in Fig. (6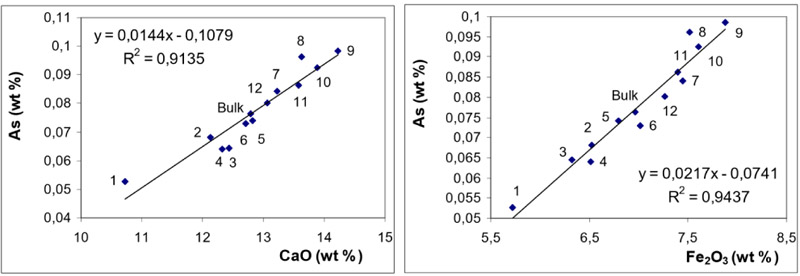 ) for FA fractions.
) for FA fractions.
While the behaviour of S (in BA and FA) is quite straightforward (indicating dominant affinity to CaO), the distribution of As exhibits good correlations with both CaO and Fe2O3. It can be seen from Figs. (5 and 6
and 6 ) that in BA, the correlation with CaO is more significant while in FA, the correlation coefficient for Fe2O3 is slightly higher.
) that in BA, the correlation with CaO is more significant while in FA, the correlation coefficient for Fe2O3 is slightly higher.
In order to distinguish between CaO and Fe2O3 associations, non-magnetic / magnetic fractions of BA and FA were analyzed for target compounds and elements (Table 3). For easier comparison and evaluation, the concentrations of the key compounds and elements are plotted in (Fig. 7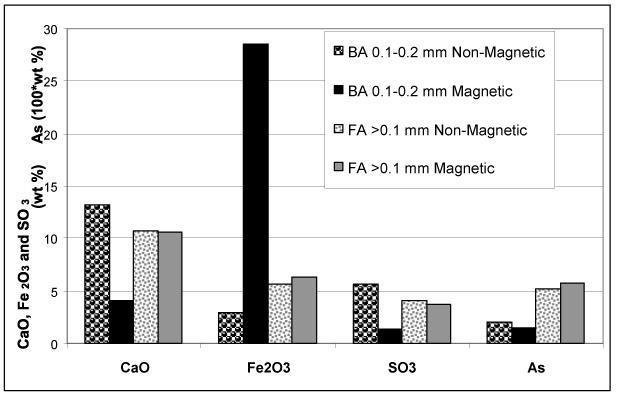 ).
).
The composition of magnetic concentrates and the residues after magnetic separation Table 3, Fig. (7 ) show (as expected) that higher concentrations of CaO were present in non-magnetic fractions and higher concentrations of Fe2O3 in magnetic fractions in both BA and FA. Data summarized in Table 3 and depicted in Fig. (7
) show (as expected) that higher concentrations of CaO were present in non-magnetic fractions and higher concentrations of Fe2O3 in magnetic fractions in both BA and FA. Data summarized in Table 3 and depicted in Fig. (7 ) reveal that SO3 exhibits higher concentrations of fractions with higher CaO content. However, the behaviour of As differed in BA and FA; in BA it followed the distribution of CaO while in FA higher concentration, was related to the fraction with higher Fe2O3 (and lower CaO) content.
) reveal that SO3 exhibits higher concentrations of fractions with higher CaO content. However, the behaviour of As differed in BA and FA; in BA it followed the distribution of CaO while in FA higher concentration, was related to the fraction with higher Fe2O3 (and lower CaO) content.
The observations revealed from magnetic / non-magnetic fractions are consistent with those inferred from the correlation coefficients: in BA As exhibits dominant association with CaO while in FA the affinity to Fe2O3 is slightly more significant.
The association between As and CaO was described (i.a.) by Clemense et al. [30Clemens, A.H.; Damiano, L.F.; Gong, D.; Metheson, T.W. Partitioning behaviour of some toxic volatile elements during stoker and fluidised-bed combustion of alkaline sub-bituminous coal. Fuel, 1999, 78, 1379-1385.
[http://dx.doi.org/10.1016/S0016-2361(99)00066-6] ], López-Antón et al. [34López-Antón, M.A; Díaz-Somoano, M.; Spears, D.A.; Martínez-Tarazona, M.R. Arsenic and Selenium Capture by Fly Ashes at Low temperature. Environ. Sci. Technol., 2006, 40, 39473951.] or Liu et al. [35Liu, H.; Wang, Ch.; Sun, X.; Zhang, Y.; Zou, Ch. Volatilization of arsenic in coal during isothermal oxyFuel combustion. Energy Fuels, 2016, 30, 3479-3487.
[http://dx.doi.org/10.1021/acs.energyfuels.6b00057] ]. The most abundant specie resulting from As-Ca interaction is Ca3(AsO4)2 [36Zhou, C.; Liu, G.; Wang, X.; Qi, C.; Hu, Y. Combustion characteristics and arsenic retention during co-combustion of agricultural biomass and bituminous coal. Bioresour. Technol., 2016, 214, 218-224.
[http://dx.doi.org/10.1016/j.biortech.2016.04.104] [PMID: 27136608] -39Wang, J.; Tomita, A. A chemistry of the volatility of some trace elements during coal combustion and pyrolysis. Energy Fuels, 2003, 17, 954-960.
[http://dx.doi.org/10.1021/ef020251o] ] and the corresponding reaction equations (3) and (4) are as follows [30Clemens, A.H.; Damiano, L.F.; Gong, D.; Metheson, T.W. Partitioning behaviour of some toxic volatile elements during stoker and fluidised-bed combustion of alkaline sub-bituminous coal. Fuel, 1999, 78, 1379-1385.
[http://dx.doi.org/10.1016/S0016-2361(99)00066-6] , 33Low, F.; Zhang, L. Arsenic emissions and speciation in the oxy-fuel fly ash collected from lab-scale droptube furnace. Proc. Combust. Inst., 2013, 34, 2877-2884.
[http://dx.doi.org/10.1016/j.proci.2012.05.026] , 40Sterling, R.O.; Helble, J.J. Reaction of arsenic vapor species with fly ash compounds: Kinetics and speciation of the reaction with calcium silicates. Chemosphere, 2003, 51(10), 1111-1119.
[http://dx.doi.org/10.1016/S0045-6535(02)00722-1] [PMID: 12718977] -42Song, Ch.; Xu, D.; Jiang, C.; Teng, Y.; Sun, Z.; An, L. The effect of particle size and metal contents in arsenic distribution in coal-fired fly ash. J. Therm. Anal. Calorim., 2014, 116, 1279-1284.
[http://dx.doi.org/10.1007/s10973-014-3684-8] ]:
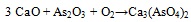 |
(3) |
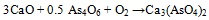 |
(4) |
Thermodynamic equilibrium calculations revealed that FeAsO4 was dominant stable specie during As-Fe interaction [32Contreras, M.L.; Arostegui, J.M.; Armesto, L. Arsenic interactions during co-combustion processes based on thermodynamic equilibrium calculations. Fuel, 2009, 88, 539-546.
[http://dx.doi.org/10.1016/j.fuel.2008.09.028] ]. Possible chemical reactions between As and Fe were presented in equation (5) (e.g.) by López-Antón et al. [11López-Antón, M.A.; Díaz-Somoano, M.; Fierro, J.L.G.; Martínez-Tarazona, M.R. Retention of arsenic and selenium compounds present in coal combustion and gasification flue gases using activated carbons. Fuel Process. Technol., 2007, 88, 799-805.
[http://dx.doi.org/10.1016/j.fuproc.2007.03.005] ].
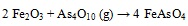 |
(5) |
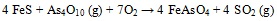 |
(6) |
 |
Fig. (7) Distribution of As within particle-size fractions of BA and FA. |
The latest detailed review on As behaviour and retention during coal combustion (published in 2018) concluded that both Fe and Ca could provide sites for As association [43Wang, Ch.; Liu, H.; Zhang, Y.; Zou, Ch.; Anthony, E.J. Review of arsenic behavior during coal combustion: Volatilization, transformation, emission and removal technologies. Pror. Energy Combust. Sci., 2018, 68, 1-28.
[http://dx.doi.org/10.1016/j.pecs.2018.04.001] ], which is consistent with the results presented in Figs. (5 and 6
and 6 ) and with similar reactivities of As to Ca and Fe reported by Seames and Wendt [31Seames, W.S.; Wendt, J.O.L. Regimes of association of arsenic and selenium during pulverized coal combustion. Proc. Combust. Inst., 2007, 31, 2839-2846.
) and with similar reactivities of As to Ca and Fe reported by Seames and Wendt [31Seames, W.S.; Wendt, J.O.L. Regimes of association of arsenic and selenium during pulverized coal combustion. Proc. Combust. Inst., 2007, 31, 2839-2846.
[http://dx.doi.org/10.1016/j.proci.2006.08.066] ] (provided both active sites are available).
However, there is no generally supported conclusion which As association is more significant and which conditions are preferred. For example, laboratory-scale As retention experiments with various Fe and/or Ca contents [45Zhang, K.; Zhang, D.; Zhang, K.; Cao, Y. Capture of gas-phase arsenic by ferrospheres separated from fly ashes. Energy Fuels, 2016, 30, 8746-8752.
[http://dx.doi.org/10.1021/acs.energyfuels.6b01637] -47Zhang, Y.; Wang, Ch.; Li, W.; Liu, H.; Zhang, Y.; Hack, P.; Pan, W. Removal of gas-phase As2O3 by metal oxide adsorbents: Effects of experimental conditions and evaluation of adsorption machanism. Energy Fuels, 2015, 29, 6578-6585.
[http://dx.doi.org/10.1021/acs.energyfuels.5b00948] ] lead to the conclusion that As was preferentially associated with Fe. Herein, Figs. (5 and 6
and 6 ) indicate that preferential association of As to Fe was observed only in FA. It is interesting to note in this context that according to Zielinski et al. [44Zielinski, R.A.; Foster, A.L.; Meeker, G.P.; Brownfield, I.K. Mode of occurrence of arsenic in feed coal and its derivative fly ash, Black Warrior Basin, Alabama. Fuel, 2007, 86, 560-572.
) indicate that preferential association of As to Fe was observed only in FA. It is interesting to note in this context that according to Zielinski et al. [44Zielinski, R.A.; Foster, A.L.; Meeker, G.P.; Brownfield, I.K. Mode of occurrence of arsenic in feed coal and its derivative fly ash, Black Warrior Basin, Alabama. Fuel, 2007, 86, 560-572.
[http://dx.doi.org/10.1016/j.fuel.2006.07.033] ], important affecting parameter is alkalinity/acidity of the studied ash - alkaline ashes promote As-Ca association whereas As-Fe association prevails in acidic ashes. Zhou et al. [46Zhou, Ch.; Liu, G.; Xu, Z.; Sun, H.; Lam, P.K.S. Effect of ash composition on the partitioning of arsenic during fluidised bed combustion. Fuel, 2017, 204, 91-97.
[http://dx.doi.org/10.1016/j.fuel.2017.05.048] ] concluded predominant As-Fe association during laboratory-scale experiments where similar concentrations of Fe and Ca were used, which is quite different from the conditions in this study. Herein (Figs. 5 and 6
and 6 ), the concentrations of CaO in (bulk) BA and FA was nearly the same (12.84 and 12.79%) whereas the contents of Fe2O3 in BA and FA was 3.33% and 6.96% (there was a twofold increase in FA in comparison with BA). Better availability of Fe active sites in FA might have resulted in less significant As-Ca association. Since the magnetic fraction of FA contains also higher MnO content, this effect cannot be excluded as well. Nevertheless, it is interesting to note that the significance of As-Ca and As-Fe associations is BA and FA is still comparable, despite the fact that the content of Fe2O3 in BA and FA is significantly lower than that of CaO.
), the concentrations of CaO in (bulk) BA and FA was nearly the same (12.84 and 12.79%) whereas the contents of Fe2O3 in BA and FA was 3.33% and 6.96% (there was a twofold increase in FA in comparison with BA). Better availability of Fe active sites in FA might have resulted in less significant As-Ca association. Since the magnetic fraction of FA contains also higher MnO content, this effect cannot be excluded as well. Nevertheless, it is interesting to note that the significance of As-Ca and As-Fe associations is BA and FA is still comparable, despite the fact that the content of Fe2O3 in BA and FA is significantly lower than that of CaO.
Temperature is another important parameter affecting As retention [37Jadhav, R.A.; Fan, L-S. Capture of gas-phase arsenic oxide by lime: Kinetic and mechanistic studies. Environ. Sci. Technol., 2001, 35(4), 794-799.
[http://dx.doi.org/10.1021/es001405m] [PMID: 11349294] ]. The adsorption of As on FA components is believed to be a combination of chemical and physical adsorption. Even though the effect of temperature is quite complicated, it is widely accepted that the dominant process in higher temperature regions is chemisorption [43Wang, Ch.; Liu, H.; Zhang, Y.; Zou, Ch.; Anthony, E.J. Review of arsenic behavior during coal combustion: Volatilization, transformation, emission and removal technologies. Pror. Energy Combust. Sci., 2018, 68, 1-28.
[http://dx.doi.org/10.1016/j.pecs.2018.04.001] ]. However, the thermodynamically predicted behaviour can occur only if there is no kinetic limitation (e.g., rapid flue gas cooling) [40Sterling, R.O.; Helble, J.J. Reaction of arsenic vapor species with fly ash compounds: Kinetics and speciation of the reaction with calcium silicates. Chemosphere, 2003, 51(10), 1111-1119.
[http://dx.doi.org/10.1016/S0045-6535(02)00722-1] [PMID: 12718977] , 48Yudovich, Ya.E.; Ketris, M.P. Arsenic in coal: A review. Int. J. Coal Geol., 2005, 61, 141-196.
[http://dx.doi.org/10.1016/j.coal.2004.09.003] ]. Therefore, this effect can be another possible reason of the differences in As behaviour in BA and FA.
In addition to the aforementioned reasons, grain-size effect should be mentioned in this context as well. Nevertheless, typical physical condensation on the finest FA particles is quite unlikely in these samples because As concentrations in the finest FA fractions are lower than those in medium size fractions.
CONCLUSION
Possible relationships between the concentrations of S and As and the contents of CaO and Fe2O3 were evaluated in BA and FA fractions from a fluidised-bed power station.
The dominant association of SO3 was with CaO, which corresponds with efficient desulphurization using desulphurization additive, such as limestone. In contrast, As exhibited association with both CaO and Fe2O3 - CaO correlation was more significant in BA while Fe2O3 correlation slightly prevailed in FA. The difference in As behaviour in BA and FA could correspond (e.g.) with bulk ash composition (concentration of Fe2O3 in FA is twofold higher than that in BA) or grain-size effect.
The results document that not only (widely accepted) Ca-bearing additives but also Fe-based ash components can be used for efficient decreasing toxic As emissions from coal-fired power stations.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The author declares no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The research was financially supported by Regional Materials Science and Technology Centre (LO 1203).
REFERENCES
| [1] | Raclavská, H.; Juchelková, D.V.; Roubíček, V.; Matysek, D. Energy utilization of biowaste-sunflower seed hulls for co-firing with coal. Fuel Process. Technol., 2011, 92, 13-20. [http://dx.doi.org/10.1016/j.fuproc.2010.03.006] |
| [2] | Raclavská, H.; Juchelková, D.; Škrobánková, H.; Wiltowski, T.; Campen, A. Conditions for energy generation as an alternative approach to compost utilization. Environ. Technol., 2011, 32(3-4), 407-417. [http://dx.doi.org/10.1080/09593330.2010.501089] [PMID: 21780708] |
| [3] | Wagner, N.J.; Hlatshwayo, B. The occurrence of potentially hazardous trace elements in five Highveld coals, South Africa. Int. J. Coal Geol., 2005, 63, 228-246. [http://dx.doi.org/10.1016/j.coal.2005.02.014] |
| [4] | Wagner, N.J.; Tlotleng, M.T. Distribution of selected trace elements in density fractionated Waterberg coals from South Africa. Int. J. Coal Geol., 2012, 94, 225-237. [http://dx.doi.org/10.1016/j.coal.2012.01.005] |
| [5] | Klika, Z.; Bartoňová, L.; Spears, D.A. Effect of boiler output on trace element partitioning during coal combustion in two fluidised-bed power stations. Fuel, 2001, 80, 907-917. [http://dx.doi.org/10.1016/S0016-2361(00)00164-2] |
| [6] | Wagner, N.J. The characterization of weathered discard coals and their behaviour during combustion. Fuel, 2008, 87, 1687-1697. [http://dx.doi.org/10.1016/j.fuel.2007.09.009] |
| [7] | Germani, M.S.; Zoller, W.H. Vapor-phase concentrations of arsenic, selenium, bromine, iodine, and mercury in the stack of a coal-fired power plant. Environ. Sci. Technol., 1988, 22(9), 1079-1085. [http://dx.doi.org/10.1021/es00174a013] [PMID: 22148663] |
| [8] | Meij, R.; Janssen, L.H.J.M.; Van Der Kooij, J. Air pollutant emissions from coal-fired power stations. Kema Scientific & Technical Reports, 1986, 4, 51-69. |
| [9] | Scala, F.; Chirone, R.; Lancia, A. Elemental mercury vapor capture by powdered activated carbon in a fluidised bed reactor. Fuel, 2011, 90, 2077-2082. [http://dx.doi.org/10.1016/j.fuel.2011.02.042] |
| [10] | Vidic, R.D.; McLaughlin, J.B. Uptake of elemental mercury vapors by activated carbons. J. Air Waste Manag. Assoc., 1996, 46(3), 241-250. [http://dx.doi.org/10.1080/10473289.1996.10467458] [PMID: 28065140] |
| [11] | López-Antón, M.A.; Díaz-Somoano, M.; Fierro, J.L.G.; Martínez-Tarazona, M.R. Retention of arsenic and selenium compounds present in coal combustion and gasification flue gases using activated carbons. Fuel Process. Technol., 2007, 88, 799-805. [http://dx.doi.org/10.1016/j.fuproc.2007.03.005] |
| [12] | Hower, J.; Thomas, G.A.; Clifford, D.S.; Eady, J.D. Petrography and Chemistry of High-Carbon Fly Ash form the Shawnee Power Station, Kentucky. Energy Sources, 1996, 18, 107118. [http://dx.doi.org/10.1080/00908319608908751] |
| [13] | Wagner, N.J.; Matjie, R.H.; Slaghuis, J.H.; Van Heerden, J.H.P. Characterization of unburned carbon present in coarse gasification ash. Fuel, 2008, 87, 683-691. [http://dx.doi.org/10.1016/j.fuel.2007.05.022] |
| [14] | Raclavská, H.; Hlavsová, A.; Juchelková, D.; Zajonc, O. The geochemical properties of carbon black from pyrolysis of municipal waste. Inzynieria Mineralna, 2013, 1, 19-27. |
| [15] | Hower, J.C.; Maroto-Valer, M.; Taulbee, D.N.; Sakulpitakphon, T. Mercury capture by distinct fly ash carbon forms. Energy Fuels, 2000, 14, 224-226. [http://dx.doi.org/10.1021/ef990192n] |
| [16] | Kostova, I.; Hower, J.C.; Mastarlerz, M.; Vassilev, S.V. Mercury capture by selected Bulgarian fly ashes: Influence of coal rank and fly ash carbon pore structure on capture efficiency. Appl. Geochem., 2011, 26, 18-27. [http://dx.doi.org/10.1016/j.apgeochem.2010.10.009] |
| [17] | Hower, J.C.; Senior, C.L.; Suuberg, E.M.; Hurt, R.H.; Wilcox, J.L.; Olson, E.S. Mercury capture by native fly ash carbons in coal-fired power plants. Pror. Energy Combust. Sci., 2010, 36(4), 510-529. [http://dx.doi.org/10.1016/j.pecs.2009.12.003] [PMID: 24223466] |
| [18] | Bartoňová, L. Unburned carbon from coal combustion: An overview. Fuel Process. Technol., 2015, 134, 136-158. [http://dx.doi.org/10.1016/j.fuproc.2015.01.028] |
| [19] | Hower, J.C.; Trimble, A.S.; Eble, C.F.; Palmer, C.A.; Kolker, A. Charakterization of fly ash from low-sulphur and high-sulphur coal sources: Partitioning of carbon and trace elements with particle size. Energy Sources, 1999, 21, 511-525. [http://dx.doi.org/10.1080/00908319950014641] |
| [20] | Bartonová, L.; Čech, B.; Ruppenthalová, L.; Majvelderová, V.; Juchelková, D.; Klika, Z. Effect of unburned carbon content in fly ash on the retention of 12 elements out of coal-combustion flue gas. J. Environ. Sci. (China), 2012, 24(9), 1624-1629. [http://dx.doi.org/10.1016/S1001-0742(11)60981-9] [PMID: 23520870] |
| [21] | Bartoňová, L.; Klika, Z.; Spears, D.A. Characterization of unburned carbon from ash after bituminous coal and lignite combustion in CFBs. Fuel, 2007, 86, 455-463. [http://dx.doi.org/10.1016/j.fuel.2006.07.024] |
| [22] | Hower, J.C.; Groppo, J.G.; Graham, U.M.; Ward, C.R.; Kostova, I.J.; Maroto-Valer, M.M.; Dai, S. Coalderived unburned carbons in fly ash: A review. Int. J. Coal Geol., 2017, 179, 11-27. [http://dx.doi.org/10.1016/j.coal.2017.05.007] |
| [23] | Bartoňová, L.; Ruppenthalová, L.; Ritz, M. Adsorption of Naphthol Green B on unburned carbon: 2- and 3 parameter linear and non-linear equilibrium modelling. Chin. J. Chem. Eng., 2017, 25, 37-44. [http://dx.doi.org/10.1016/j.cjche.2016.05.035] |
| [24] | Wendt, J.O.L.; Lee, S.J. High-temperature sorbents for Hg, Cd, Pb and other trace metals: Mechanisms and applications. Fuel, 2010, 89, 894-903. [http://dx.doi.org/10.1016/j.fuel.2009.01.028] |
| [25] | Scala, F.; Anacleria, C.; Cimino, S. Characterization of a regenerable sorbent for high temperature elemental mercury capture from flue gas. Fuel, 2013, 108, 13-18. [http://dx.doi.org/10.1016/j.fuel.2010.12.028] |
| [26] | Scala, F.; Cimino, S. Elemental mercury capture and oxidation by a regenerable manganese-based sorbent: The effect of gas composition. Chem. Eng. J., 2015, 278, 134-139. [http://dx.doi.org/10.1016/j.cej.2014.11.094] |
| [27] | Scala, F.; Chirone, R.; Meloni, P.; Carcangiu, G.; Manca, M.; Mulas, G.; Mulas, A. Fluidised bed desulphurization using lime obtained after slow calcination of limestone particles. Fuel, 2013, 114, 99105. [http://dx.doi.org/10.1016/j.fuel.2012.11.072] |
| [28] | Raclavská, H.; Matysek, D.; Raclavský, K.; Juchelková, D. Geochemistry of fly ash from desulphurisation process performed by sodium bicarbonate. Fuel Process. Technol., 2010, 91, 150-157. [http://dx.doi.org/10.1016/j.fuproc.2009.09.004] |
| [29] | Bartoňová, L.; Klika, Z. Effect of CaO on retention of S, Cl, Br, As, Mn, V, Cr, Ni, Cu, Zn, W and Pb in bottom ashes from fluidized-bed coal combustion power station. J. Environ. Sci. (China), 2014, 26(7), 1429-1436. [http://dx.doi.org/10.1016/j.jes.2014.05.008] [PMID: 25079991] |
| [30] | Clemens, A.H.; Damiano, L.F.; Gong, D.; Metheson, T.W. Partitioning behaviour of some toxic volatile elements during stoker and fluidised-bed combustion of alkaline sub-bituminous coal. Fuel, 1999, 78, 1379-1385. [http://dx.doi.org/10.1016/S0016-2361(99)00066-6] |
| [31] | Seames, W.S.; Wendt, J.O.L. Regimes of association of arsenic and selenium during pulverized coal combustion. Proc. Combust. Inst., 2007, 31, 2839-2846. [http://dx.doi.org/10.1016/j.proci.2006.08.066] |
| [32] | Contreras, M.L.; Arostegui, J.M.; Armesto, L. Arsenic interactions during co-combustion processes based on thermodynamic equilibrium calculations. Fuel, 2009, 88, 539-546. [http://dx.doi.org/10.1016/j.fuel.2008.09.028] |
| [33] | Low, F.; Zhang, L. Arsenic emissions and speciation in the oxy-fuel fly ash collected from lab-scale droptube furnace. Proc. Combust. Inst., 2013, 34, 2877-2884. [http://dx.doi.org/10.1016/j.proci.2012.05.026] |
| [34] | López-Antón, M.A; Díaz-Somoano, M.; Spears, D.A.; Martínez-Tarazona, M.R. Arsenic and Selenium Capture by Fly Ashes at Low temperature. Environ. Sci. Technol., 2006, 40, 39473951. |
| [35] | Liu, H.; Wang, Ch.; Sun, X.; Zhang, Y.; Zou, Ch. Volatilization of arsenic in coal during isothermal oxyFuel combustion. Energy Fuels, 2016, 30, 3479-3487. [http://dx.doi.org/10.1021/acs.energyfuels.6b00057] |
| [36] | Zhou, C.; Liu, G.; Wang, X.; Qi, C.; Hu, Y. Combustion characteristics and arsenic retention during co-combustion of agricultural biomass and bituminous coal. Bioresour. Technol., 2016, 214, 218-224. [http://dx.doi.org/10.1016/j.biortech.2016.04.104] [PMID: 27136608] |
| [37] | Jadhav, R.A.; Fan, L-S. Capture of gas-phase arsenic oxide by lime: Kinetic and mechanistic studies. Environ. Sci. Technol., 2001, 35(4), 794-799. [http://dx.doi.org/10.1021/es001405m] [PMID: 11349294] |
| [38] | Shen, F.; Liu, J.; Zhang, Z.; Dai, J. On-Line analysis and kinetic behavior of arsenic release during coal combustion and pyrolysis. Environ. Sci. Technol., 2015, 49(22), 13716-13723. [http://dx.doi.org/10.1021/acs.est.5b03626] [PMID: 26488499] |
| [39] | Wang, J.; Tomita, A. A chemistry of the volatility of some trace elements during coal combustion and pyrolysis. Energy Fuels, 2003, 17, 954-960. [http://dx.doi.org/10.1021/ef020251o] |
| [40] | Sterling, R.O.; Helble, J.J. Reaction of arsenic vapor species with fly ash compounds: Kinetics and speciation of the reaction with calcium silicates. Chemosphere, 2003, 51(10), 1111-1119. [http://dx.doi.org/10.1016/S0045-6535(02)00722-1] [PMID: 12718977] |
| [41] | Chen, D.; Hu, H.; Xu, Z.; Liu, H.; Cao, J.; Shen, J.; Yao, H. Findings of proper temperatures for arsenic capture by CaO in the simulated flue gas with and without SO2. Chem. Eng. J., 2015, 267, 201-206. [http://dx.doi.org/10.1016/j.cej.2015.01.035] |
| [42] | Song, Ch.; Xu, D.; Jiang, C.; Teng, Y.; Sun, Z.; An, L. The effect of particle size and metal contents in arsenic distribution in coal-fired fly ash. J. Therm. Anal. Calorim., 2014, 116, 1279-1284. [http://dx.doi.org/10.1007/s10973-014-3684-8] |
| [43] | Wang, Ch.; Liu, H.; Zhang, Y.; Zou, Ch.; Anthony, E.J. Review of arsenic behavior during coal combustion: Volatilization, transformation, emission and removal technologies. Pror. Energy Combust. Sci., 2018, 68, 1-28. [http://dx.doi.org/10.1016/j.pecs.2018.04.001] |
| [44] | Zielinski, R.A.; Foster, A.L.; Meeker, G.P.; Brownfield, I.K. Mode of occurrence of arsenic in feed coal and its derivative fly ash, Black Warrior Basin, Alabama. Fuel, 2007, 86, 560-572. [http://dx.doi.org/10.1016/j.fuel.2006.07.033] |
| [45] | Zhang, K.; Zhang, D.; Zhang, K.; Cao, Y. Capture of gas-phase arsenic by ferrospheres separated from fly ashes. Energy Fuels, 2016, 30, 8746-8752. [http://dx.doi.org/10.1021/acs.energyfuels.6b01637] |
| [46] | Zhou, Ch.; Liu, G.; Xu, Z.; Sun, H.; Lam, P.K.S. Effect of ash composition on the partitioning of arsenic during fluidised bed combustion. Fuel, 2017, 204, 91-97. [http://dx.doi.org/10.1016/j.fuel.2017.05.048] |
| [47] | Zhang, Y.; Wang, Ch.; Li, W.; Liu, H.; Zhang, Y.; Hack, P.; Pan, W. Removal of gas-phase As2O3 by metal oxide adsorbents: Effects of experimental conditions and evaluation of adsorption machanism. Energy Fuels, 2015, 29, 6578-6585. [http://dx.doi.org/10.1021/acs.energyfuels.5b00948] |
| [48] | Yudovich, Ya.E.; Ketris, M.P. Arsenic in coal: A review. Int. J. Coal Geol., 2005, 61, 141-196. [http://dx.doi.org/10.1016/j.coal.2004.09.003] |