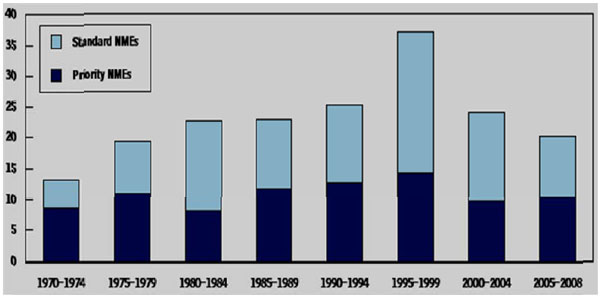
Fig. (1) Average Annual Approvals of New Drugs by the Food and Drug Administration, 1970 to 2008. The data, for new molecular
entities (NMEs) only, exclude extensions and new approved uses of existing drugs. NMEs are drugs based on a molecule not used before in
any pharmaceutical product. Priority drugs are those that, according to the Food and Drug Administration, provide a “significant therapeutic
or public health advance.” (Source: Congressional Budget Office based on data from the FDA.)