- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

The Open Nutrition Journal
(Discontinued)
ISSN: 1874-2882 ― Volume 15, 2021
Daily Oral Chondroitin Sulfate Oligosaccharides for Knee Joint Pain in Healthy Subjects: A Randomized, Blinded, Placebo-Controlled Study
Mie Nishimura1, Nobuyuki Miyamoto2, Jun Nishihira1, *
Abstract
Background:
The increased rate of population aging in Japan has led to an increase in the incidence of osteoarthritis (OA). Chondroitin sulfate has been reported to reduce the pain and swelling associated with OA and to improve knee function.
Objective:
We evaluated the safety and effects of oral chondroitin sulfate oligosaccharides on knee function in a randomized, double-blinded, placebo-controlled parallel group comparison study of healthy Japanese subjects with knee joint pain.
Methods:
Subjects were randomly divided into test and placebo groups and given either active-test capsules containing 100 mg of chondroitin sulfate oligosaccharides or placebo capsules daily for 8 weeks. The Japanese Knee Osteoarthritis Measure (JKOM), Visual Analog Scale (VAS), blood and physical examinations, and medical interviews were performed at weeks 0, 4, and 8, and the locomotive syndrome risk test was performed at weeks 0 and 8 during the test intake period.
Results:
The JKOM scores did not significantly differ between the test groups. However, among subjects with worse VAS scores, those in the active test group had significantly lower JKOM scores at 8 weeks, compared to those in the placebo group. Moreover, chondroitin sulfate oligosaccharide treatment tended to improve the subjects' scores on the stand-up test, which evaluates the risk of locomotive syndrome. Furthermore, no abnormal changes or severe adverse events were observed during physical or blood examinations or medical interviews.
Conclusion:
Our results suggest that chondroitin sulfate oligosaccharides improve knee pain and are safe for 8-week intake.
Article Information
Identifiers and Pagination:
Year: 2018Volume: 12
First Page: 10
Last Page: 20
Publisher Id: TONUTRJ-12-10
DOI: 10.2174/1874288201812010010
Article History:
Received Date: 13/02/2018Revision Received Date: 5/05/2018
Acceptance Date: 06/05/2018
Electronic publication date: 31/05/2018
Collection year: 2018
open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
* Address correspondence to the author at the Department of Medical Management and Informatics, Hokkaido Information University, Nishi Nopporo 59-2, Ebetsu 069-8585 Hokkaido, Japan; Tel: +81-011-385-4430; E-mail: nishihira@do-johodai.ac.jp
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 13-02-2018 |
Original Manuscript | Daily Oral Chondroitin Sulfate Oligosaccharides for Knee Joint Pain in Healthy Subjects: A Randomized, Blinded, Placebo-Controlled Study | |
1. INTRODUCTION
Japan is currently experiencing population aging, and the related changes have led to an increase in the incidence of osteoarthritis (OA). OA is a localized pain and movement disorder caused by cartilage destruction. The knee is most frequently affected by OA, as the load borne by this joint is heavy relative to the loads of other joints. Currently, 25 million people are estimated to suffer from knee OA [1Muraki S, Oka H, Akune T, et al. Prevalence of radiographic knee osteoarthritis and its association with knee pain in the elderly of Japanese population-based cohorts: the ROAD study. Osteoarthritis Cartilage 2009; 17(9): 1137-43.
[http://dx.doi.org/10.1016/j.joca.2009.04.005] [PMID: 19410032] ]. This condition causes pain, and deteriorating symptoms limit the patient's ability to walk and perform lifestyle activities. Accordingly, the onset of knee OA negatively impacts the “quality of life” of aging people. In addition, The Japanese Orthopedic Association has suggested the concept of “locomotive syndrome” [2Nakamura K. A “super-aged” society and the “locomotive syndrome”. J Orthop Sci 2008; 13(1): 1-2.
[http://dx.doi.org/10.1007/s00776-007-1202-6] [PMID: 18274847] , 3Nakagata T, Ozaki H, Machida S, Ishibashi M, Naito H. Effect of long-term training program combining increased physical activity and walking with blood flow restriction on locomotive syndrome in the elderly. Juntendo Med J 2016; 62(Suppl. 1): 211-7.
[http://dx.doi.org/10.14789/jmj.62.s211] ], wherein a patient with movement disorder either requires or is at a high risk of requiring long-term care. Notably, knee OA has been identified as a risk factor for locomotive syndrome.
Currently, people expect that functional foods aimed at improving knee function will become available. Chondroitin, a cartilage proteoglycan component and dietary supplement, is found in cartilage from sharks or rays [4Gokan N, Suzuki N, Shiizuka K, Yamamoto K, Takara T. The Effect of the dietary supplement containing with both glucosamine and chondroitin sulfate on knee joint pain. J New Rem & Clin 2011; 60(7): 1476-82.]. Chondroitin sulfate was previously found to decrease pain and swelling and improve knee function [5Uebelhart D. Clinical review of chondroitin sulfate in osteoarthritis. Osteoarthritis Cartilage 2008; 16(Suppl. 3): S19-21.
[http://dx.doi.org/10.1016/j.joca.2008.06.006] [PMID: 18674931] , 6Hochberg MC, Clegg DO. Potential effects of chondroitin sulfate on joint swelling: a GAIT report. Osteoarthritis Cartilage 2008; 16(Suppl. 3): S22-4.
[http://dx.doi.org/10.1016/j.joca.2008.06.024] [PMID: 18768335] ] and was shown to exert anti-inflammatory effects in an in vitro assay based on chondrocytes and synoviocytes [7Iovu M, Dumais G, du Souich P. Anti-inflammatory activity of chondroitin sulfate. Osteoarthritis Cartilage 2008; 16(Suppl. 3): S14-8.
[http://dx.doi.org/10.1016/j.joca.2008.06.008] [PMID: 18667340] ]. Another report demonstrated that chondroitin sulfate promoted the production of glycosaminoglycans such as hyaluronic acid and chondroitin [8Ishida K, Nakatani S, Kobata K, Wada M. Effect of chondroitin sulphate for the proliferation and differentiation of prechondrocyte, ATDC5 (in Japanese). Chitin chitosan Res 2011; 17(2): 244.]. However, chondroitin sulfate has a high molecular weight, which limits its absorption from the intestine. Therefore, the successful industrial production of chondroitin sulfate oligosaccharides derived from cartilage requires the use of a subcritical and supercritical water hydrolysis method [9Yamada S, Matsushima K, Ura H, Miyamoto N, Sugahara K. Mass preparation of oligosaccharides by the hydrolysis of chondroitin sulfate polysaccharides with a subcritical water microreaction system. Carbohydrate Res 2013; 371: 16-21.
[http://dx.doi.org/10.1016/j.carres.2013.01.024] [PMID: 23454651] ]. In an in vitro everted gut technique, this low-molecular-weight form of chondroitin was absorbed at the level of several hundred times higher than that of high-molecular-weight chondroitin.
In this 8-week randomized, double-blinded, placebo-controlled, parallel group comparison study, we assessed the effects of chondroitin sulfate oligosaccharides on the severity of knee pain and the risk of locomotive syndrome in 60 healthy subjects (aged 45-68 years) with knee pain.
2. MATERIALS AND METHODS
2.1. Study Subjects
For this clinical study, we recruited 98 volunteers with knee joint pain, all of whom provided written informed consent to participate. Through screening tests, we finally selected 60 healthy Japanese subjects (aged 45–68 years) with subjective symptoms of knee joint pain that did not require hospital treatment, which was determined by a physician. The following volunteers were excluded: (1) individuals receiving treatment/medication for OA; (2) injured subjects who faced problems with daily life activities; (3) subjects receiving treatment and medication for rheumatoid arthritis (RA) or who were suspected to have RA; (4) subjects suspected to have secondary arthrosis; (5) subjects who regularly used anticoagulants, antiplatelet agents, or non-steroidal anti-inflammatory drugs (NSAIDs); (6) those with serious cerebrovascular, cardiac, hepatic, renal, or gastrointestinal disease; (7) those with a history of major surgery related to the digestive system; (8) those with unusually abnormal hematological data or serious anemia; (9) those who regularly used medicines, functional foods, and/or supplements that would improve knee joint function; (10) heavy smokers, alcohol addicts, or subjects with irregular lifestyles; (11) those with a severe allergic reaction to medicine or foods; (12) those who are pregnant or expected to be pregnant, or lactating during the study; and (13) those with other medical reasons as determined by the principal investigator.
The 60 remaining eligible subjects were randomly assigned to the active test food or placebo food groups. A third-party data center assigned these subjects to the aforementioned groups, stratified by sex, age, and the level of the sum score of questions 2-5 on the Japanese Knee Osteoarthritis Measure (JKOM) [10Akai M, Doi T, Fujino K, Iwaya T, Kurosawa H, Nasu T. An outcome measure for Japanese people with knee osteoarthritis. J Rheumatol 2005; 32(8): 1524-32.
[PMID: 16078330] ]. The allocations were computer generated using stratified block randomization. Doctors, nurses, clinical research coordinators, and statistical analyzers had no knowledge of the assignment information during this trial period. This information was only disclosed after the laboratory and analytical data were fixed and the method of statistical analysis was finalized.
2.2. Study Design
This clinical study was conducted as a randomized, double-blinded, placebo-controlled parallel group comparison study at the Hokkaido Information University, Health Information Science Research Center (Ebetsu city, Hokkaido, Japan) in 2016 according to the general clinical trial design for assessing the efficacy of functional food [11Nishimura M, Sugawara M, Kudo M, Nishihira J. A randomized, double-blind, placebo-controlled study to examine the effects of high-isoflavone soybeans “Yukipirika” in climacteric women. Funct Food Health Dis 2017; 7(8): 637-60.]. The examination schedule for this study is shown in Table 1. We performed JKOM and Visual Analog Scale (VAS) questionnaires at weeks 0 (baseline), 4, and 8. A locomotive syndrome risk analysis was performed at weeks 0 and 8 after initiating the test food-intake period. A medical interview and physical, hematological, and biological examinations were performed at weeks 0, 4, and 8. We asked all subjects to consume two capsules of a test food containing either 100 mg of chondroitin sulfate oligosaccharides or placebo. During this study, subjects were asked not to change their daily activities, including food consumption and exercise habits and medication use, and to record a life diary during the study period. The primary outcome was the JKOM score, and the secondary outcome was the VAS questionnaire score and locomotive syndrome risk analysis.
2.3. Preparation of Test Food
Chondroitin sulfate oligosaccharides were manufactured as follows: raw high-molecular-weight chondroitin sulfate was obtained by liquefying ray cartilage using protease, removing the peptide, and powdering the extract via spray drying. The powdered high-molecular-weight chondroitin sulfate was dissolved in deionized water and reacted continuously at 213ºC. Thereafter, the reaction solution was cooled, concentrated, and purified using filter pores to remove impurities. This purified solution was then powdered by spray drying to obtain a chondroitin sulfate oligosaccharide powder, which was then placed in capsules (active test food). The placebo food was manufactured according to the same procedure, except that fried wheat was used instead of chondroitin sulfate oligosaccharides. The results of the nutrient composition analyses of the active test food and placebo food used in this study are provided in Table 2. The nutrient composition analyses were performed by the Japan Food Research Laboratories (Chitose, Japan). Chondroitin sulfate oligosaccharides were analyzed using High-Performance Liquid Chromatography (HPLC) methods by Marukyou Suisan Co. Ltd. The active test food and placebo food were manufactured under strict quality control protocols by Marukyou Suisan Co. Ltd. and were identical in appearance.
2.4. JKOM
To evaluate the effects of chondroitin sulfate oligosaccharides on knee joint function, all subjects completed the JKOM, which comprises five sections: (1) Scale of knee pain; (2) Questions regarding the experienced knee pain and stiffness; (3) Questions regarding the state of the subject's daily life; (4) Questions regarding daily activities; and (5) Questions regarding the subject's health status. The subjects answered Question 1 (JKOM 1) by placing a cross on a 100-mm line where the left end of (0 mm) was defined as the best condition and the right end (100 mm) as the worst condition. The responses to this question were assessed by evaluating the length from the left-hand start of the line to the cross. A decrease in the JKOM 1 score indicated an improvement. For questions 2-5 (JKOM 2-5), the subjects selected one of five answers (0 points = best condition; 4 points = worst condition). The maximum possible score was 200 points, and a reduction in the JKOM 2-5 score indicated an improvement in knee joint function.
2.5. VAS Questionnaire
To evaluate the effects of chondroitin sulfate oligosaccharides on knee pain, the subjects completed a VAS questionnaire comprising three questions to assess (1) knee pain at rest, (2) knee pain at walking, and (3) knee pain while climbing up and down stairs. Subjects were instructed to mark a cross on a 100-mm line in response to each question based on their current health condition. Here, the left end of the line (0 mm) was defined as the worst condition and the right end (100 mm) as the best condition. The questionnaire results were assessed by evaluating the length from the left-hand start of the line to the cross. An increase in the VAS score indicated an improvement in that symptom.
2.6. Locomotive Syndrome Risk Test
The locomotive syndrome risk test comprised three parts: (1) a questionnaire regarding locomotive syndrome, (2) the two-steps test, and (3) the stand-up test [12 Otsuka Pharmaceutical Co. L. Locomotive Syndrome Rsik Test [Available from: https://www.otsuka.co.jp/en/health_illness/locomo/, 13Conference JLCP. Conference JLCP. Locomotive syndrome [Available from: https://locomo-joa.jp/en/index.pdf]. The locomotive syndrome questionnaire comprised 25 questions for the assessment of body pain and other factors. A decrease in this questionnaire score indicated an improvement. The two-step test measures walking ability including muscular strength, balance, and flexibility of the lower limbs. Subjects took two long strides, the longest they could without losing their balance. This test was performed twice, and the better result was recorded. The two-step score was calculated by dividing the length of both strides (cm) by the subject's height. The stand-up test assesses leg strength by having the subject stand up on one or both legs. Subjects stand up from each seat, starting at 40 cm, first with one leg. If subjects could stand up, and maintain the position for 3 seconds, next, they were made to stand up at 30 cm with one leg. If subjects could not stand up, they were made to stand up with both legs from the same-height seat which they could not stand up with one leg. The high score was of the following order: 10 cm with one leg, 20 cm with one leg, 30 cm with one leg, 40 cm with one leg, 10 cm with both legs, 20 cm with both legs, 30 cm with both legs, and 40 cm with both legs. We compared the 8-week score to the 0-week score, and evaluated “worse”, “no-change” and “improvement”.
2.7. Physical, Hematological, and Biological Measurements
Blood was collected after a 12-h fast. General blood tests were performed, including the measurement of complete blood counts (CBCs; white blood cells, red blood cells, hemoglobin, hematocrit, and platelets), liver function (aspartate aminotransferase, alanine aminotransferase, gamma glutamyl transpeptidase, alkaline phosphatase, and lactate dehydrogenase), and renal function (blood urea nitrogen, creatinine, and uric acid). Blood tests were performed by the Sapporo Clinical Laboratory, Inc. (Sapporo, Japan).
Each subject’s body composition and blood pressure were measured using a Body Composition Analyzer DC-320 (Tanita Corp, Tokyo, Japan) and an Automatic Blood Pressure Monitor HEM-7080IC (Omron Co., Ltd., Kyoto, Japan) respectively.
2.8. Ethics Committee
All subjects provided written informed consent prior to undergoing any of the tests related to this study. The Ethics Committee of Hokkaido Information University approved the study protocol conforming to the Helsinki Declaration and Ethical Guidelines for Medical and Health Research Involving Human Subjects (approval date, Jul 21, 2016; approval number 2016-04). This study was registered with UMIN Clinical Trials Registry (approval number UMIN000023492).
2.9. Sample Size
The sample size was statistically determined to obtain a power of 80% and a two-sided significance level of 5%. To demonstrate JKOM 2-5 at week 8, which was postulated to have an intergroup difference of 10 points with a standard deviation (SD) of 12 points, a sample size of 54 (27 in each group) was required. Assuming a 10% loss in the follow-up rate, 60 subjects were selected.
2.10. Statistical Analysis
Values are presented in the tables as means ± standard deviations. Changes in subject values were analyzed using Mann-Whitney U test for evaluation of JKOM, VAS, and the questionnaire of locomotive syndrome, and Student’s t test for the evaluation of two-step test between active test food and placebo food at each evaluation point without statistical consideration for multiplicity. Frequency values were obtained with chi-square test for the evaluation of the stand-up test. p value <0.05 was considered to be significant. Statistical analyses were performed using SPSS Statistics 20 (IBM, Armonk, NY, USA).
3. RESULTS
3.1. Subject Dropouts, Exclusions, and Characteristics
Although we initially enrolled 60 subjects (active test food, n = 30, placebo food, n = 30), two subjects withdrew for personal reasons before the trial began. Additionally, two subjects withdrew during the trial (abnormal liver function value before test food intake, n = 1; injury, n = 1). Consequently, 56 subjects completed the trial (placebo food group, n = 26; active test food group, n = 30). No subject was excluded from efficacy analysis (placebo food group, n = 26; active test food group, n = 30). The subjects who ingested food even once were included in the safety analysis (placebo food group, n = 28; active test food group, n = 30). The study flow diagram is shown in Fig. (1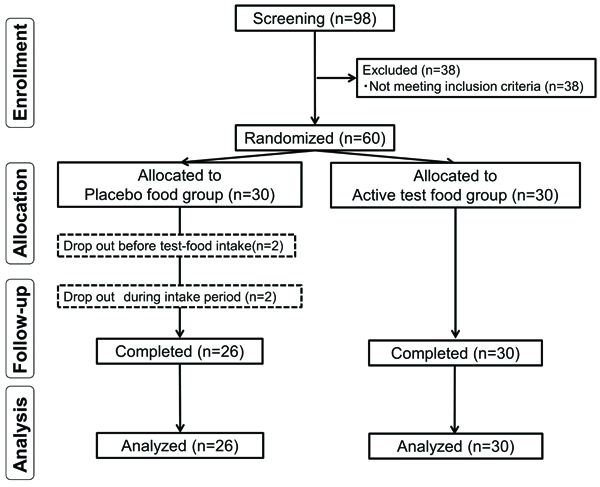 ). Table 3 presents the mean age, height, body weight, body mass index, score for JKOM 1, and summed score for JKOM 2-5 for each group. These data did not differ significantly between the groups, which confirmed the appropriateness of subject allocation. Moreover, the active test food and placebo food groups did not differ regarding the intake rate (Table 3).
). Table 3 presents the mean age, height, body weight, body mass index, score for JKOM 1, and summed score for JKOM 2-5 for each group. These data did not differ significantly between the groups, which confirmed the appropriateness of subject allocation. Moreover, the active test food and placebo food groups did not differ regarding the intake rate (Table 3).
 |
Fig. (1) Flow diagram of the trial. |
Values are shown as means ± standard deviations. Student’s t test was used for analyses of age, height, body weight, and body mass index, and the chi-square test was used to evaluate sex. The Mann-Whitney U test was used to analyze JKOM scores and the intake rate. n, number of subjects.
3.2. Effects of Chondroitin Sulfate Oligosaccharides on JKOM Scores
We evaluated the effects of chondroitin sulfate oligosaccharides on JKOM scores (Table 4), but observed no differences between the active test food and placebo food groups regarding changes in JKOM. However, among subjects with worse VAS scores (using the median baseline total VAS score for stratification), the sum of JKOM scores 2–5 improved significantly in the active test food group relative to the placebo food group at weeks 8 (change from baseline to week 8: placebo, −3.85 ± 9.09 points; active, −11.33 ± 6.58 points, p = 0.016). Moreover, the JKOM score 1 tended to improve with active test food intake relative to the placebo at week 4 (change from baseline to week 4: placebo, −3.69 ± 20.18 mm; active, −18.40 ± 16.86 mm, p = 0.07).
3.3. Effects of Chondroitin Sulfate Oligosaccharides on VAS scores
Next, we confirmed the effects of chondroitin sulfate oligosaccharides on knee pain. We found no differences in the changes in VAS scores between the active test food and placebo food groups (Table 4).
3.4. Effects of Chondroitin Sulfate Oligosaccharides on the Locomotive Syndrome Risk Test
To evaluate the effects of chondroitin sulfate oligosaccharides on locomotive syndrome, the subjects completed the questionnaire of locomotive syndrome, two-steps test, and stand-up test. The results of stand-up test exhibited a differing trend between the active test food group and placebo food group (p = 0.054) (Table 5). Next, to confirm whether the frequencies of the subjects’ score to the stand-up test differed between the two groups, we performed a residual analysis, which revealed that “worse” was significantly reduced in the active test food-intake group, compared to the placebo food group (adjusted residual: ±2.2). Although the active test food group exhibited improvements in the questionnaire of locomotive syndrome and two-steps test outcomes, these differences were not significant (Table 5).
 week 8, change from baseline to week 8. Values are shown as means ± standard deviations for the questionnaire of locomotive syndrome and two-steps test, and as frequencies for the stand-up test. The mean changes in subject values at each evaluation point were compared between the two groups using the Mann-Whitney U test for the questionnaire of locomotive syndrome and Student’s t test. The frequency values from the stand-up test were compared using the chi-square test.
week 8, change from baseline to week 8. Values are shown as means ± standard deviations for the questionnaire of locomotive syndrome and two-steps test, and as frequencies for the stand-up test. The mean changes in subject values at each evaluation point were compared between the two groups using the Mann-Whitney U test for the questionnaire of locomotive syndrome and Student’s t test. The frequency values from the stand-up test were compared using the chi-square test.
3.5. Safety
We further evaluated blood pressure, body composition, CBCs, and liver and renal function after the subjects ingested the test foods (Table 6). Only minimal changes within the normal ranges were observed. Moreover, few subjects exhibited adverse effects, and these effects were mild and resolved within a few days. Therefore, the principal investigator determined that no adverse events were related to the ingestion of the test food. Additionally, no abnormal changes or severe adverse events were observed during the physical, hematological, and biological examinations or the medical interview. Accordingly, the 8-week intake of 100 mg of low molecular chondroitin sulfate was considered safe.
4. DISCUSSION
In this study, we have demonstrated the beneficial effects of chondroitin sulfate oligosaccharides for the treatment of knee pain in otherwise healthy subjects. Although the JKOM scores did not significantly differ between subjects who received the test food and those receiving the placebo, among subjects with worse VAS scores, those in the active test food group had significantly lower summed JKOM 2-5 scores, compared to those in the placebo food group, at week 8. Moreover, chondroitin sulfate oligosaccharide treatment tended to decrease the risk of locomotive syndrome, as indicated by the stand-up test scores.
A previous study reported a strong correlation between the JKOM and VAS scores [14Watanabe H, Urabe K, Kamiya K, et al. Relationship between quality of life using the japan knee osteoarthritis measure (JKOM) and physical function in patients with osteoarthritis of the knee. J Jpn Phys Ther Assoc 2007; 34(3): 67-73.]. In our trial, the baseline JKOM and VAS scores were strongly correlated (Pearson correlation coefficient, -0.533**). These results would suggest that chondroitin sulfate oligosaccharides are more effective in subjects with relatively severe knee pain. However, our clinical trial did not include the subjects who require hospitalization for treatment. Indeed, the average of JKOM score was lower in our clinical trial. These results suggest that chondroitin sulfate oligosaccharides were more efficient for subjects who have symptoms of knee pain but do not need treatment. In addition, in our clinical trial, the VAS questionnaire scores did not differ between the two groups. We note that the JKOM solicited more specific details regarding knee pain, such as the duration or situation, in comparison to the VAS. This might explain why we did not observe improved VAS scores. In addition, the intake periods was short compared to the previous trials which VAS score was improved by test food intake [15Kahan A, Uebelhart D, De Vathaire F, Delmas PD, Reginster JY. Long-term effects of chondroitins 4 and 6 sulfate on knee osteoarthritis: the study on osteoarthritis progression prevention, a two-year, randomized, double-blind, placebo-controlled trial. Arthritis Rheum 2009; 60(2): 524-33.
[http://dx.doi.org/10.1002/art.24255] [PMID: 19180484] ]. We need to re-evaluate our intake period.
Locomotive syndrome is defined as a state of mobility disability caused by a failure of the exerciser, and risk factors for this syndrome include a reduction in balance ability or muscle strength, bone disease [16Muramoto A, Imagama S, Ito Z, Hirano K, Ishiguro N, Hasegawa Y. Spinal sagittal balance substantially influences locomotive syndrome and physical performance in community-living middle-aged and elderly women. J Orthop Sci 2016; 21(2): 216-21.
[http://dx.doi.org/10.1016/j.jos.2015.12.016] [PMID: 26806334] , 17Nakamura K, Yoshimura N, Ogata T, Akune T, Tobimatsu Y. The concept of locomotive syndrome and its relationship with frailty and sarcopenia. Nihon Rinsho 2015; 73(10): 1746-53.
[PMID: 26529941] ], and arthritic disease [18Chosa E. Locomotive syndrome and frailty. Locomotive syndrome due to the underlying disease of degenerative arthritis. Clin Calcium 2012; 22(4): 49-57.
[PMID: 22460511] ]. It appears that chondroitin prevented locomotive syndrome by improving the subjects’ knee pain. In addition, to clarify the effects of BW on the locomotive syndrome risk test, we divided the subjects into two subgroups: normal BW subject group and high-normal/class I obesity subject groups. In subjects with normal BW (placebo, n = 19; active, n = 12), the stand-up test results differed significantly between the active test food and placebo food groups (p = 0.024), and the frequency of “improvement” was significantly higher in the active test food group in a residual analysis (adjusted residual: ±2.4). We further observed that the locomotive syndrome questionnaire scores improved significantly improved with active test food intake ( 8 week, placebo food group: -2.42 ± 6.75 points vs. active test food group: -7.00 ± 6.34 points, p = 0.048). According to a previous report, knee pain in female subjects is associated with the body mass index and dietary habits [19Sato S, Nemoto Y, Takahashi M, et al. The relevant factors for knee pain in community-dwelling elderly: A cross-sectional study. Nippon Koshu Eisei Zasshi 2016; 63(9): 560-8.
8 week, placebo food group: -2.42 ± 6.75 points vs. active test food group: -7.00 ± 6.34 points, p = 0.048). According to a previous report, knee pain in female subjects is associated with the body mass index and dietary habits [19Sato S, Nemoto Y, Takahashi M, et al. The relevant factors for knee pain in community-dwelling elderly: A cross-sectional study. Nippon Koshu Eisei Zasshi 2016; 63(9): 560-8.
[PMID: 27818469] ]. These results suggest that improved knee pain requires not only the ingestion of an active test food but also the maintenance of a normal body weight.
Finally, we note that we observed no abnormal changes or severe adverse events during the physical and blood examinations or medical interviews during this clinical trial. Therefore, our results confirm the safety of 100 mg of low-molecular-weight chondroitin sulfate oligosaccharides for an 8-week period.
CONCLUSION
In this 8-week randomized, double-blinded, placebo-controlled, parallel group comparison study, we determined the effects of chondroitin sulfate oligosaccharides relative to a placebo in healthy subjects with knee pain. Notably, chondroitin sulfate oligosaccharide supplementation improved the JKOM scores of subjects with relatively severe knee pain, and had positive effects on the locomotive syndrome risk test outcomes. Additionally, we confirmed the safety of the 8-week intake of 100.0 mg of chondroitin sulfate oligosaccharides. Although the biological mechanism underlying these results requires further elucidation, our findings support the use of chondroitin sulfate oligosaccharides as a new functional food for the treatment of knee pain.
LIST OF ABBREVIATIONS
| ALT | = Alanine Aminotransferase |
| ALP | = Alkaline Phosphatase |
| AST | = Aspartate Aminotransferase |
| BP | = Blood Pressure |
| BUN | = Blood Urea Nitrogen |
| BFP | = Body Fat Percentage |
| BMI | = Body Mass Index |
| BW | = Body Weight |
| CBCs | = Complete Blood Counts |
| CRE | = Creatinine |
| DBP | = Diastolic Blood Pressure |
| γ-GTP | = Gamma Glutamyl Transpeptidases |
| Ht | = Hematocrit |
| Hb | = Hemoglobin |
| JKOM | = Japanese Knee Osteoarthritis Measure |
| LDH | = Lactate Dehydrogenase |
| Plt | = Platelet |
| RBC | = Red blood cells |
| SBP | = Systolic Blood Pressure |
| UA | = Uric Acid |
| VAS | = Visual Analog Scale |
| WBC | = White Blood Cells |
AUTHORS’ CONTRIBUTIONS
JN, MN, and NM designed the research. NM provided the test foods. JN conducted the research. MN performed the statistical analyses. MN and JN wrote the manuscript. JN assumed primary responsibility for the final content. All authors read and approved the final version of the manuscript.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The Ethics Committee of Hokkaido Information University approved the study protocol (approval date, Jul 21, 2016; approval number 2016-04). This study was registered with UMIN Clinical Trials Registry (approval number UMIN000023492).
HUMAN AND ANIMAL RIGHTS
The Ethics Committee of Hokkaido Information University approved the study protocol conforming to the Helsinki Declaration and Ethical Guidelines for Medical and Health Research Involving Human Subjects.
CONSENT FOR PUBLICATION
All subjects provided written informed consent prior to undergoing any of the tests related to this study.
CONFLICT OF INTEREST
This study was funded by Marukyou Suisan Co. Ltd. The conflicts of interest were evaluated and approved by the Hokkaido Information University ethics review committee.
ACKNOWLEDGEMENTS
We thank the members of the Center of Health Information Science at Hokkaido Information University (Anzai Y., Fukuda Y., Ito M., Kamo S., Koyama S., Miyao A., Obata N., Ohkubo Y., Ohshima M., Saito T., Saito Y, Sasaki M., Sato K., Shima N., Tanaka A., Teramoto M., and Tsunemine S.) for their technical assistance with the clinical trial.
REFERENCES
| [1] | Muraki S, Oka H, Akune T, et al. Prevalence of radiographic knee osteoarthritis and its association with knee pain in the elderly of Japanese population-based cohorts: the ROAD study. Osteoarthritis Cartilage 2009; 17(9): 1137-43. [http://dx.doi.org/10.1016/j.joca.2009.04.005] [PMID: 19410032] |
| [2] | Nakamura K. A “super-aged” society and the “locomotive syndrome”. J Orthop Sci 2008; 13(1): 1-2. [http://dx.doi.org/10.1007/s00776-007-1202-6] [PMID: 18274847] |
| [3] | Nakagata T, Ozaki H, Machida S, Ishibashi M, Naito H. Effect of long-term training program combining increased physical activity and walking with blood flow restriction on locomotive syndrome in the elderly. Juntendo Med J 2016; 62(Suppl. 1): 211-7. [http://dx.doi.org/10.14789/jmj.62.s211] |
| [4] | Gokan N, Suzuki N, Shiizuka K, Yamamoto K, Takara T. The Effect of the dietary supplement containing with both glucosamine and chondroitin sulfate on knee joint pain. J New Rem & Clin 2011; 60(7): 1476-82. |
| [5] | Uebelhart D. Clinical review of chondroitin sulfate in osteoarthritis. Osteoarthritis Cartilage 2008; 16(Suppl. 3): S19-21. [http://dx.doi.org/10.1016/j.joca.2008.06.006] [PMID: 18674931] |
| [6] | Hochberg MC, Clegg DO. Potential effects of chondroitin sulfate on joint swelling: a GAIT report. Osteoarthritis Cartilage 2008; 16(Suppl. 3): S22-4. [http://dx.doi.org/10.1016/j.joca.2008.06.024] [PMID: 18768335] |
| [7] | Iovu M, Dumais G, du Souich P. Anti-inflammatory activity of chondroitin sulfate. Osteoarthritis Cartilage 2008; 16(Suppl. 3): S14-8. [http://dx.doi.org/10.1016/j.joca.2008.06.008] [PMID: 18667340] |
| [8] | Ishida K, Nakatani S, Kobata K, Wada M. Effect of chondroitin sulphate for the proliferation and differentiation of prechondrocyte, ATDC5 (in Japanese). Chitin chitosan Res 2011; 17(2): 244. |
| [9] | Yamada S, Matsushima K, Ura H, Miyamoto N, Sugahara K. Mass preparation of oligosaccharides by the hydrolysis of chondroitin sulfate polysaccharides with a subcritical water microreaction system. Carbohydrate Res 2013; 371: 16-21. [http://dx.doi.org/10.1016/j.carres.2013.01.024] [PMID: 23454651] |
| [10] | Akai M, Doi T, Fujino K, Iwaya T, Kurosawa H, Nasu T. An outcome measure for Japanese people with knee osteoarthritis. J Rheumatol 2005; 32(8): 1524-32. [PMID: 16078330] |
| [11] | Nishimura M, Sugawara M, Kudo M, Nishihira J. A randomized, double-blind, placebo-controlled study to examine the effects of high-isoflavone soybeans “Yukipirika” in climacteric women. Funct Food Health Dis 2017; 7(8): 637-60. |
| [12] | Otsuka Pharmaceutical Co. L. Locomotive Syndrome Rsik Test [Available from: https://www.otsuka.co.jp/en/health_illness/locomo/ |
| [13] | Conference JLCP. Conference JLCP. Locomotive syndrome [Available from: https://locomo-joa.jp/en/index.pdf |
| [14] | Watanabe H, Urabe K, Kamiya K, et al. Relationship between quality of life using the japan knee osteoarthritis measure (JKOM) and physical function in patients with osteoarthritis of the knee. J Jpn Phys Ther Assoc 2007; 34(3): 67-73. |
| [15] | Kahan A, Uebelhart D, De Vathaire F, Delmas PD, Reginster JY. Long-term effects of chondroitins 4 and 6 sulfate on knee osteoarthritis: the study on osteoarthritis progression prevention, a two-year, randomized, double-blind, placebo-controlled trial. Arthritis Rheum 2009; 60(2): 524-33. [http://dx.doi.org/10.1002/art.24255] [PMID: 19180484] |
| [16] | Muramoto A, Imagama S, Ito Z, Hirano K, Ishiguro N, Hasegawa Y. Spinal sagittal balance substantially influences locomotive syndrome and physical performance in community-living middle-aged and elderly women. J Orthop Sci 2016; 21(2): 216-21. [http://dx.doi.org/10.1016/j.jos.2015.12.016] [PMID: 26806334] |
| [17] | Nakamura K, Yoshimura N, Ogata T, Akune T, Tobimatsu Y. The concept of locomotive syndrome and its relationship with frailty and sarcopenia. Nihon Rinsho 2015; 73(10): 1746-53. [PMID: 26529941] |
| [18] | Chosa E. Locomotive syndrome and frailty. Locomotive syndrome due to the underlying disease of degenerative arthritis. Clin Calcium 2012; 22(4): 49-57. [PMID: 22460511] |
| [19] | Sato S, Nemoto Y, Takahashi M, et al. The relevant factors for knee pain in community-dwelling elderly: A cross-sectional study. Nippon Koshu Eisei Zasshi 2016; 63(9): 560-8. [PMID: 27818469] |