- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

The Open Nutrition Journal
(Discontinued)
ISSN: 1874-2882 ― Volume 15, 2021
Cardiomyopathy Secondary to Selenium Deficiency: A Review of Clinical Cases
Nigel Amankwah1, Zhiyong Han2, *
Abstract
Background:
Selenium is an essential micronutrient for the human body because it is needed for the synthesis of selenoproteins, which have various biological functions. As a result, selenium deficiency associated with diets and/or environments manifests in different disease states such as epilepsy, multiminicore disease and cardiovascular injury which in some cases is a presage of cardiomyopathy.
Objective:
This objective was to review published cases and identify selenium-responsive cardiomyopathy due to selenium deficiency by various factors.
Methods:
Published case reports in English were identified and extracted from PubMed, Scopus, Embase, and Science Direct Library.
Results:
28 case reports met inclusion criteria out of an initial 189 articles.
Conclusion:
Acquired selenium deficiency is a causative factor for the development of cardiomyopathy in patients under different conditions, and treatment of these patients with selenium is effective in normalizing cardiac function or reducing cardiac dysfunction. Thus, it is important to include selenium deficiency as a possible cause of cardiomyopathy for diagnosis and treatment purposes.
Article Information
Identifiers and Pagination:
Year: 2018Volume: 12
First Page: 74
Last Page: 88
Publisher Id: TONUTRJ-12-74
DOI: 10.2174/1874288201812010074
Article History:
Received Date: 20/7/2018Revision Received Date: 22/9/2018
Acceptance Date: 27/9/2018
Electronic publication date: 18/10/2018
Collection year: 2018
open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Correspondence: Address correspondence to the author at the Department of Medical Sciences, Hackensack Meridian School of Medicine at Seton Hall University, Building 123, 340 Kingsland Street, Nutley, NJ 07110, USA; Tel: 973-275-4309; E-mail: zhiyong.han@shu.edu
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 20-7-2018 |
Original Manuscript | Cardiomyopathy Secondary to Selenium Deficiency: A Review of Clinical Cases | |
1. INTRODUCTION
Selenium is an essential micronutrient for the human body [1Small-Howard AL, Berry MJ. Unique features of selenocysteine incorporation function within the context of general eukaryotic translational processes. Biochemical Society Transactions Portland Press Limited 2005; 33(): 1493-7.] since it is required for the synthesis of selenocysteine (Sec), which is needed for the synthesis of selenoproteins. Selenoproteins have important pleiotropic biological activities including antioxidant activity, anti-inflammatory activity, and deiodinase activity (which is required for the synthesis of active thyroid hormone) [2Berry MJ, Tujebajeva RM, Copeland PR, et al. Selenocysteine incorporation directed from the 3'UTR: Characterization of eukaryotic EFsec and mechanistic implications. Biofactors 2001; 14(1-4): 17-24.
[http://dx.doi.org/10.1002/biof.5520140104] [PMID: 11568436] ]. The process of selenoprotein synthesis involves numerous steps. It begins with serylation of tRNASec by seryl-tRNA synthetase to yield seryl-tRNASec. The seryl-tRNASec is phosphorylated by a phosphoseryl-tRNASec kinase to yield phosphoseryl(Sep)-tRNASec. The Sec-tRNASec is synthesized by Sep-tRNA:Sec-tRNA synthase using the phosphoseryl-tRNASec and selenophosphate [1Small-Howard AL, Berry MJ. Unique features of selenocysteine incorporation function within the context of general eukaryotic translational processes. Biochemical Society Transactions Portland Press Limited 2005; 33(): 1493-7.]. The Sec-tRNASec acts as the Sec donor for the incorporation of Sec into a nascent selenoprotein, as determined by a Sec-specific UGA codon, during translation [3Bellinger FP, Raman AV, Reeves MA, Berry MJ. Regulation and function of selenoproteins in human disease. Biochem J 2009; 422(1): 11-22.
[http://dx.doi.org/10.1042/BJ20090219] [PMID: 19627257] ].
The prototype selenoprotein is GPx (glutathione peroxidase), which was characterized as a selenocysteine in 1978 [4Forstrom JW, Zakowski JJ, Tappel AL. Identification of the catalytic site of rat liver glutathione peroxidase as selenocysteine. Biochem 1978; 17(13): 2639-44.
[http://dx.doi.org/10.1021/bi00606a028] [PMID: 678534] ]. Since, other selenoproteins with important biological functions have been discovered, including thioredoxin reductases (TRxR) [5Arnér ESJ, Holmgren A. Physiological functions of thioredoxin and thioredoxin reductase. Eur J Biochem 2000; 267(20): 6102-9.
[http://dx.doi.org/10.1046/j.1432-1327.2000.01701.x] [PMID: 11012661] ], methionine sulfoxide reductase A [6Moskovitz J. Methionine sulfoxide reductases: Ubiquitous enzymes involved in antioxidant defense, protein regulation, and prevention of aging-associated diseases. Biochim Biophys Acta 2005; 1703(2): 213-9.
[http://dx.doi.org/10.1016/j.bbapap.2004.09.003] [PMID: 15680229] ], deiodinases [7Bianco AC, Kim BW. Deiodinases: Implications of the local control of thyroid hormone action. J Clin Invest 2006; 116(10): 2571-9.
[http://dx.doi.org/10.1172/JCI29812] [PMID: 17016550] ], selenoprotein P [8Schomburg L, Schweizer U, Holtmann B, Flohé L, Sendtner M, Köhrle J. Gene disruption discloses role of selenoprotein P in selenium delivery to target tissues. Biochem J 2003; 370(Pt 2): 397-402.
[http://dx.doi.org/10.1042/bj20021853] [PMID: 12521380] ], selenoprotein N [9Hwang DY, Cho JS, Oh JH, et al. Differentially expressed genes in transgenic mice carrying human mutant presenilin-2 (N141I): Correlation of selenoprotein M with Alzheimer’s disease. Neurochem Res 2005; 30(8): 1009-19.
[http://dx.doi.org/10.1007/s11064-005-6787-6] [PMID: 16258850] ], selenoprotein M [10Zorzato F, Jungbluth H, Zhou H, Muntoni F, Treves S. Functional effects of mutations identified in patients with multiminicore disease. IUBMB Life 2007; 59(1): 14-20.
[http://dx.doi.org/10.1080/15216540601187803] [PMID: 17365175] ], selenoprotein T [11Grumolato L, Ghzili H, Montero-Hadjadje M, et al. Selenoprotein T is a PACAP-regulated gene involved in intracellular Ca2+ mobilization and neuroendocrine secretion. FASEB J 2008; 22(6): 1756-68.
[http://dx.doi.org/10.1096/fj.06-075820] [PMID: 18198219] ] and selenoprotein S [12Ye Y, Shibata Y, Yun C, Ron D, Rapoport TA. A membrane protein complex mediates retro-translocation from the ER lumen into the cytosol. Nature 2004; 429(6994): 841-7.
[http://dx.doi.org/10.1038/nature02656] [PMID: 15215856] ]. The important functions of representative selenoproteins are listed in Table 1. Furthermore, studies of animals and humans have linked deficiencies of various selenoproteins and polymorphisms in selenoprotein genes with a medley of diseases including muscle and cardiovascular disorders, hypothyroidism, and epilepsy [3Bellinger FP, Raman AV, Reeves MA, Berry MJ. Regulation and function of selenoproteins in human disease. Biochem J 2009; 422(1): 11-22.
[http://dx.doi.org/10.1042/BJ20090219] [PMID: 19627257] ].
There is a clinical association between selenium deficiency and epilepsy [3Bellinger FP, Raman AV, Reeves MA, Berry MJ. Regulation and function of selenoproteins in human disease. Biochem J 2009; 422(1): 11-22.
[http://dx.doi.org/10.1042/BJ20090219] [PMID: 19627257] ]. Thus, infants with intractable epilepsy have significant lower levels of selenium compared with the normal control group (p < 0.05) [13Ashrafi MR, Shabanian R, Abbaskhanian A, et al. Selenium and intractable epilepsy: Is there any correlation? Pediatr Neurol 2007; 36(1): 25-9.
[http://dx.doi.org/10.1016/j.pediatrneurol.2006.09.001] [PMID: 17162193] ]. In animal studies, it has been demonstrated that a selenium deficient diet increased the susceptibility of rats to kainite-induced epileptic seizures [14Savaskan NE, Bräuer AU, Kühbacher M, et al. Selenium deficiency increases susceptibility to glutamate-induced excitotoxicity. FASEB J 2003; 17(1): 112-4.
[http://dx.doi.org/10.1096/fj.02-0067fje] [PMID: 12424220] ], and neurological seizures were observed in selenoprotein P knockout mice when they were raised on selenium restricted diet [8Schomburg L, Schweizer U, Holtmann B, Flohé L, Sendtner M, Köhrle J. Gene disruption discloses role of selenoprotein P in selenium delivery to target tissues. Biochem J 2003; 370(Pt 2): 397-402.
[http://dx.doi.org/10.1042/bj20021853] [PMID: 12521380] , 15Hill KE, Zhou J, McMahan WJ, et al. Deletion of selenoprotein P alters distribution of selenium in the mouse. J Biol Chem 2003; 278(16): 13640-6.
[http://dx.doi.org/10.1074/jbc.M300755200] [PMID: 12574155] ].
Multiminicore, a form of congenital muscular dystrophy, characterized by a distinct loss of muscle fiber organization, has been associated with selenium deficiency and/or selenoprotein defects [16Jungbluth H. Multi-minicore disease. Orphanet J Rare Dis 2007; 2: 31.
[http://dx.doi.org/10.1186/1750-1172-2-31] [PMID: 17631035] ]. The etiology has been attributed to mutations in selenoprotein N and ryanodine receptors [10Zorzato F, Jungbluth H, Zhou H, Muntoni F, Treves S. Functional effects of mutations identified in patients with multiminicore disease. IUBMB Life 2007; 59(1): 14-20.
[http://dx.doi.org/10.1080/15216540601187803] [PMID: 17365175] ]. Ryanodine receptors are transmembrane proteins in the endoplasmic reticulum and are responsible for releasing calcium from the intracellular stores [17Zalk R, Lehnart SE, Marks AR. Modulation of the ryanodine receptor and intracellular calcium. Annu Rev Biochem 2007; 76: 367-85.
[http://dx.doi.org/10.1146/annurev.biochem.76.053105.094237] [PMID: 17506640] ]. It appears that selenoprotein N associates with and facilitates the ryanodine receptors to function properly [18Jurynec MJ, Xia R, Mackrill JJ, et al. Selenoprotein N is required for ryanodine receptor calcium release channel activity in human and zebrafish muscle. Proc Natl Acad Sci USA 2008; 105(34): 12485-90.
[http://dx.doi.org/10.1073/pnas.0806015105] [PMID: 18713863] ]. It was demonstrated in a zebra fish model that selenoprotein N knockout resulted in disorganized muscle fibers, the hallmark of multiminicore disease [19Deniziak M, Thisse C, Rederstorff M, Hindelang C, Thisse B, Lescure A. Loss of selenoprotein N function causes disruption of muscle architecture in the zebrafish embryo. Exp Cell Res 2007; 313(1): 156-67.
[http://dx.doi.org/10.1016/j.yexcr.2006.10.005] [PMID: 17123513] ]. Besides selenoprotein N, selenoproteins M and T also play a vital role in the regulation of calcium-mediated signaling: selenoprotein M attenuates calcium signaling in response to oxidative stress [20Reeves MA, Bellinger FP, Berry MJ. The neuroprotective functions of selenoprotein M and its role in cytosolic calcium regulation. Antioxid Redox Signal 2010; 12(7): 809-18.
[http://dx.doi.org/10.1089/ars.2009.2883] [PMID: 19769485] ], whereas, selenoprotein T increases it [11Grumolato L, Ghzili H, Montero-Hadjadje M, et al. Selenoprotein T is a PACAP-regulated gene involved in intracellular Ca2+ mobilization and neuroendocrine secretion. FASEB J 2008; 22(6): 1756-68.
[http://dx.doi.org/10.1096/fj.06-075820] [PMID: 18198219] ]. Thus, selenoproteins N, M and T are important regulators of calcium signaling relevant to muscular health and function.
Deiodinases are selenoproteins that play an essential role in thyroid hormone metabolism and function [7Bianco AC, Kim BW. Deiodinases: Implications of the local control of thyroid hormone action. J Clin Invest 2006; 116(10): 2571-9.
[http://dx.doi.org/10.1172/JCI29812] [PMID: 17016550] ]. The biologically active thyroid hormone 3,5,3′-triiodothyronine (T3) is activated by deiodinase from its precursor thyroxine (T4). Specifically, deiodinases types I and II convert T4 to T3 whereas the deiodinase type III inactivates both T4 and T3 by removing specific iodine atoms from T3 and T4 [7Bianco AC, Kim BW. Deiodinases: Implications of the local control of thyroid hormone action. J Clin Invest 2006; 116(10): 2571-9.
[http://dx.doi.org/10.1172/JCI29812] [PMID: 17016550] ]. Since thyroid hormone regulates a plethora of organs metabolic functions including the heart, dysregulation of thyroid hormone levels due to deficiencies of selenium and/or defects in deiodinases increases the risk of pathological cardiovascular perturbations [21Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. N Engl J Med 2001; 344(7): 501-9.
[http://dx.doi.org/10.1056/NEJM200102153440707] [PMID: 11172193] ].
Reactive oxygen species are known to cause injury to all cell types including the vascular endothelial cells and cardiac myocytes [22Lum H, Roebuck KA. Oxidant stress and endothelial cell dysfunction. Am J Physiol Cell Physiol 2001; 280(4): C719-41.
[http://dx.doi.org/10.1152/ajpcell.2001.280.4.C719] [PMID: 11245588] ]. GPx uses glutathione to detoxify hydroperoxides into water, thereby providing cells anti-oxidant protection [23Arthur JR. The glutathione peroxidases. Cell Mol Life Sci 2000; 57(13-14): 1825-35.
[PMID: 11215509] ]. Studies of mice showed that GPx1 inhibited ischemia induced apoptosis of cardiac myocytes [24Maulik N, Yoshida T, Das DK. Regulation of cardiomyocyte apoptosis in ischemic reperfused mouse heart by glutathione peroxidase. Mol Cell Biochem 1999; 196(1-2): 13-21.
[http://dx.doi.org/10.1023/A:1006905910140] [PMID: 10448898] ], and that targeted deletion of GPx1 gene lead to heart and vascular dysfunction [25Forgione MA, Cap A, Liao R, et al. Heterozygous cellular glutathione peroxidase deficiency in the mouse: Abnormalities in vascular and cardiac function and structure. Circulation 2002; 106(9): 1154-8.
[http://dx.doi.org/10.1161/01.CIR.0000026820.87824.6A] [PMID: 12196344] ]. Other forms of GPx, GPx3 and GPx4, provide protection against thrombosis [26Kenet G, Freedman J, Shenkman B, et al. Plasma glutathione peroxidase deficiency and platelet insensitivity to nitric oxide in children with familial stroke. Arterioscler Thromb Vasc Biol 1999; 19(8): 2017-23.
[http://dx.doi.org/10.1161/01.ATV.19.8.2017] [PMID: 10446087] ] and atherosclerosis [27Guo Z, Van Remmen H, Yang H, et al. Changes in expression of antioxidant enzymes affect cell-mediated LDL oxidation and oxidized LDL-induced apoptosis in mouse aortic cells. Arterioscler Thromb Vasc Biol 2001; 21(7): 1131-8.
[http://dx.doi.org/10.1161/hq0701.092092] [PMID: 11451741] ]. The positive correlation between selenium and GPx activities/expression alludes to selenium’s indirect cardiovascular protective role. For example selenium supplementation increases GPx1 and GPx4 activities in vascular endothelial cells, resulting in reduced oxidative stress [28Miller S, Walker SW, Arthur JR, et al. Selenite protects human endothelial cells from oxidative damage and induces thioredoxin reductase. Clin Sci (Lond) 2001; 100(5): 543-50.
[http://dx.doi.org/10.1042/cs1000543] [PMID: 11294695] -31Thomas JP, Geiger PG, Girotti AW. Lethal damage to endothelial cells by oxidized low density lipoprotein: Role of selenoperoxidases in cytoprotection against lipid hydroperoxide- and iron-mediated reactions. J Lipid Res 1993; 34(3): 479-90.
[PMID: 8468531] ]. Conversely, long term selenium deficiency leads to decreased GPx expression and activity and cardiovascular damage, which can be reversed by dietary supplementation of selenium [32Huang K, Liu H, Chen Z, Xu H. Role of selenium in cytoprotection against cholesterol oxide-induced vascular damage in rats. Atherosclerosis 2002; 162(1): 137-44.
[http://dx.doi.org/10.1016/S0021-9150(01)00707-9] [PMID: 11947907] , 33Wu Q, Huang K. Effect of selenium compounds on the damage induced by oxysterol on rat arterial walls. Biol Trace Elem Res 2006; 112(3): 273-82.
[http://dx.doi.org/10.1385/BTER:112:3:273] [PMID: 17057266] ].
TRxR is crucial for the control of cellular redox homeostasis because it detoxifies peroxides, prevents deleterious disulfide bonds formation within and between biomolecules, reduces thioredoxin, and regulates the redox state of transcription factors, thereby regulating the redox homeostasis in cells [5Arnér ESJ, Holmgren A. Physiological functions of thioredoxin and thioredoxin reductase. Eur J Biochem 2000; 267(20): 6102-9.
[http://dx.doi.org/10.1046/j.1432-1327.2000.01701.x] [PMID: 11012661] ]. Studies of mice have demonstrated that systemic inactivation of TRxR resulted in embryonic lethality associated with anemic embryos, thinning of the ventricular heart wall and malformation of the heart [34Conrad M, Jakupoglu C, Moreno SG, et al. Essential role for mitochondrial thioredoxin reductase in hematopoiesis, heart development, and heart function. Mol Cell Biol 2004; 24(21): 9414-23.
[http://dx.doi.org/10.1128/MCB.24.21.9414-9423.2004] [PMID: 15485910] ]. Mice studies also show that cardiac tissue-selective knockout of the mitochondrial TRxR gene results in fatal dilated cardiomyopathy [34Conrad M, Jakupoglu C, Moreno SG, et al. Essential role for mitochondrial thioredoxin reductase in hematopoiesis, heart development, and heart function. Mol Cell Biol 2004; 24(21): 9414-23.
[http://dx.doi.org/10.1128/MCB.24.21.9414-9423.2004] [PMID: 15485910] ]. Additionally, cardiomyocyte cell death under ischemic and reperfusion conditions due to failure of thiol regeneration have also been shown in TRxR knockout mice studies [35Horstkotte J, Perisic T, Schneider M, et al. Mitochondrial thioredoxin reductase is essential for early postischemic myocardial protection. Circulation 2011; 124(25): 2892-902.
[http://dx.doi.org/10.1161/CIRCULATIONAHA.111.059253] [PMID: 22144571] ]. In parallel to these findings, studies of humans have established the protective role of TRxR in the cardiovascular system and identified loss-of-function mutations of TrxR that cause dilated cardiomyopathy in patients [36Sibbing D, Pfeufer A, Perisic T, et al. Mutations in the mitochondrial thioredoxin reductase gene TXNRD2 cause dilated cardiomyopathy. Eur Heart J 2011; 32(9): 1121-33.
[http://dx.doi.org/10.1093/eurheartj/ehq507] [PMID: 21247928] ]. In short, loss of mitochondrial TRxR activity in the heart is a pathogenic factor for the development of dilated cardiomyopathy.
Given the fact that GPx and TRxR are selenoproteins, selenium nutrition plays an important role in the antioxidant systems in the human body. Hence deficiencies of GPx, TRxR and deoidenases secondary to selenium deficiency can result in the pathogenesis of cardiovascular diseases. Thus, the classic demonstration of diseases caused by selenium deficiency is the Keshan disease, which was originally discovered in China with clinical features that are characteristic of dilated cardiomyopathy: cardiogenic shock, cardiac arrhythmias, ECG changes, enlarged heart, and/or congestive heart failure [37Chen J. An original discovery: Selenium deficiency and Keshan disease (an endemic heart disease). Asia Pac J Clin Nutr 2012; 21(3): 320-6.
[PMID: 22705420] ]. Epidemiological and population-based intervention studies in China established a causal relationship between dietary deficiency of selenium and the development of Keshan disease [37Chen J. An original discovery: Selenium deficiency and Keshan disease (an endemic heart disease). Asia Pac J Clin Nutr 2012; 21(3): 320-6.
[PMID: 22705420] ]. Subsequent case-controlled studies in other countries, such as the one in Finland [38Salonen JT, Alfthan G, Huttunen JK, Pikkarainen J, Puska P. Association between cardiovascular death and myocardial infarction and serum selenium in a matched-pair longitudinal study. Lancet 1982; 2(8291): 175-9.
[http://dx.doi.org/10.1016/S0140-6736(82)91028-5] [PMID: 6123886] ], established an inverse relationship between the serum selenium level and incidence of myocardial infarction and cardiovascular death. Specifically, a low level of serum selenium (< 45 μg/L) was associated with 2-3 times of increased risk for death due to cardiovascular diseases [38Salonen JT, Alfthan G, Huttunen JK, Pikkarainen J, Puska P. Association between cardiovascular death and myocardial infarction and serum selenium in a matched-pair longitudinal study. Lancet 1982; 2(8291): 175-9.
[http://dx.doi.org/10.1016/S0140-6736(82)91028-5] [PMID: 6123886] ]. However, certain epidemiological features of Keshan disease could not be explained solely by selenium deficiency because there are other factors involved [39Levander OA, Beck MA. Interacting nutritional and infectious etiologies of Keshan disease. Insights from coxsackie virus B-induced myocarditis in mice deficient in selenium or vitamin E. Biol Trace Elem Res 1997; 56(1): 5-21.
[http://dx.doi.org/10.1007/BF02778980] [PMID: 9152508] ]. For example, selenium deficiency increases the susceptibility of mice to myocarditis induction by Coxsackie B virus infection [40Beck MA. Nutritionally induced oxidative stress: Effect on viral disease. Am J Clin Nutr 2000; 71(6)(Suppl.): 1676S-81S.
[http://dx.doi.org/10.1093/ajcn/71.6.1676S] [PMID: 10837315] ]. Nevertheless, it is likely that selenium deficiency predisposes an individual to Keshan disease that can be precipitated by other etiological factors and that these factors are likely to act synergistically with selenium deficiency to cause Keshan disease.
At the molecular level, it seems that the best explanation for cardiomyopathy secondary to selenium deficiency is that selenium deficiency causes myopathy as a result of the depletion of GPx and TRxR that protect cardiomyocytes from oxidative damage. Although it is intuitive that selenium deficiency can occur in individuals with malabsorption and/or malnutrition or living in a selenium-deficient environment, there has been no, to the best of our knowledge, systemic review of the published clinical case reports for etiologies of selenium deficiency in relationship to the development of cardiomyopathy. Therefore, in an effort to understand how patients who do not live in selenium deficient areas acquire selenium deficiency and develop selenium-responsive cardiomyopathy, we researched the biomedical literature to identify and review cardiomyopathy cases in which selenium deficiency plays a causative role.
2. METHODS
A systematic search of the published biomedical literature was conducted in PubMed, Scopus, Embase, and Science Direct Library databases for studies investigating cardiomyopathy associated with selenium deficiency. For optimal identification of relevant studies, the search criteria used the following keywords alone and in combination: selenium deficiency, diet, malabsorption, cardiomyopathy, and heart failure. There were no date restrictions applied to the search. For consistency, the same criteria were implemented across all databases. Study selection entailed screening for studies in which selenium deficiency played a role in the etiology of a cardiomyopathy, which was validated on the basis of cardiomyopathy symptoms resolution or reduction after repletion of selenium or selenium deficient level findings postmortem. Pertinent information from each article was recorded in Tables 2 and 3 without altercation of information or conversion of any selenium values and units reported. Non-English written articles were excluded due our inability to comprehend the language the manuscripts were composed in.
3. RESULTS
As shown in Fig. (1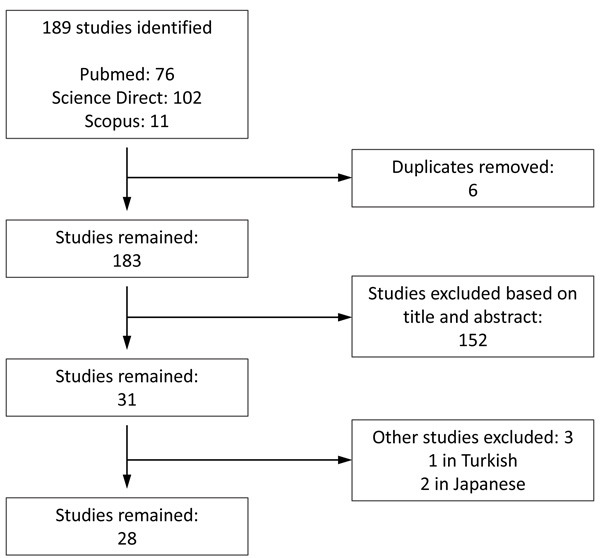 ), our search strategy yielded a total of 189 studies. We retrieved 31 studies of which 28 met our criteria and were included in our report. Tables 2 and 3 contain relevant information regarding each patient’s gender, age, cause of selenium deficiency, selenium status, symptoms that prompted selenium analysis, form of selenium used for treatment, and length of selenium treatment to resolve symptoms.
), our search strategy yielded a total of 189 studies. We retrieved 31 studies of which 28 met our criteria and were included in our report. Tables 2 and 3 contain relevant information regarding each patient’s gender, age, cause of selenium deficiency, selenium status, symptoms that prompted selenium analysis, form of selenium used for treatment, and length of selenium treatment to resolve symptoms.
 |
Fig. (1) Flowchart of study selection. |
3.1. Ketogenic Diet Causes Selenium Deficiency and Cardiomyopathy
The ketogenic diet is a low-carb diet that substantially changes the energy fuel partition in the body. The classic ketogenic diet is based on a 4:1 of fat to carbohydrate ratio [41Levy RG, Cooper PN, Giri P. Ketogenic diet and other dietary treatments for epilepsy. Cochrane Database Syst Rev 2012; 3(3): CD001903.
[PMID: 22419282] ]. The ketogenic diet has been proven to be beneficial to patients with drug-resistant epilepsy [42Perucca P, Scheffer IE, Kiley M. The management of epilepsy in children and adults. Med J Aust 2018; 208(5): 226-33.
[http://dx.doi.org/10.5694/mja17.00951] [PMID: 29540143] ]. One study showed that 38% children in the study who had been placed on ketogenic diet for three months experienced a reduction in seizures by over 50% compared to 6% in the control group [43Winesett SP, Bessone SK, Kossoff EH. The ketogenic diet in pharmacoresistant childhood epilepsy. Expert Rev Neurother 2015; 15(6): 621-8.
[http://dx.doi.org/10.1586/14737175.2015.1044982] [PMID: 25994046] ]. In the same study, 5 children in the ketogenic diet group experienced a reduction in seizures by 90% compared to the control [43Winesett SP, Bessone SK, Kossoff EH. The ketogenic diet in pharmacoresistant childhood epilepsy. Expert Rev Neurother 2015; 15(6): 621-8.
[http://dx.doi.org/10.1586/14737175.2015.1044982] [PMID: 25994046] ]. In another study, 55% children with refractory epilepsy became seizure free after they had been placed on a ketogenic diet that was 80% fat in terms of total calorie [44Seo JH, Lee YM, Lee JS, Kang HC, Kim HD. Efficacy and tolerability of the ketogenic diet according to lipid:nonlipid ratios: Comparison of 3:1 with 4:1 diet. Epilepsia 2007; 48(4): 801-5.
[http://dx.doi.org/10.1111/j.1528-1167.2007.01025.x] [PMID: 17386059] ]. However, ketogenic diet is not entirely safe; its high fat content and low carbohydrate intake may lead to hyperlipidemia, atherosclerosis, and hypoglycemia [45Azevedo de Lima P, Baldini Prudêncio M, Murakami DK, Pereira de Brito Sampaio L, Figueiredo Neto AM, Teixeira Damasceno NR. Effect of classic ketogenic diet treatment on lipoprotein subfractions in children and adolescents with refractory epilepsy. Nutrition 2017; 33: 271-7.
[http://dx.doi.org/10.1016/j.nut.2016.06.016] [PMID: 27712963] , 46Lin A, Turner Z, Doerrer SC, Stanfield A, Kossoff EH. Complications during ketogenic diet initiation: Prevalence, Treatment, and influence on seizure outcomes. Pediatr Neurol 2017; 68: 35-9.
[http://dx.doi.org/10.1016/j.pediatrneurol.2017.01.007] [PMID: 28188074] ]. In this review, we identified four cases of selenium-responsive pediatric cardiomyopathy that resulted from selenium deficiency due to the use of ketogenic diet to manage their epilepsy (Table 2). Selenium deficiency as the cause of cardiomyopathy was validated by the fact that treatment of these patients with selenium supplement normalized their cardiac functions.
3.2. Selenium Deficiency and Cardiomyopathy Associated with Gastrointestinal Disorders
Intestinal failure, due to functional or anatomic changes, results in reduced absorption of macronutrients and micronutrient below the minimum threshold level. Short Bowel Syndrome (SBS) [47Patel KS, Carroll R. Hormonal management of small bowel failure. Clin Transl Gastroenterol 2017; 8(6): e105.
[http://dx.doi.org/10.1038/ctg.2017.32] [PMID: 28662020] ] is a condition that occurs when less than 200 cm of the small intestine remains because of bowel disease, congenital defect or surgery [48Wanten G, Calder PC, Forbes A. Managing adult patients who need home parenteral nutrition. BMJ 2011; 342: d1447.
[http://dx.doi.org/10.1136/bmj.d1447] [PMID: 21421667] ]. In SBS/intestinal failure, the body is unable to absorb sufficient minerals, nutrients, water and vitamins from the diet to sustain life [47Patel KS, Carroll R. Hormonal management of small bowel failure. Clin Transl Gastroenterol 2017; 8(6): e105.
[http://dx.doi.org/10.1038/ctg.2017.32] [PMID: 28662020] ] and as a result, Parenteral Nutrition (PN) must be administered to curtail malnutrition [49Scribner BH, Cole JJ, Christopher TG, Vizzo JE, Atkins RC, Blagg CR. Long-term total parenteral nutrition. The concept of an artificial gut. JAMA 1970; 212(3): 457-63.
[http://dx.doi.org/10.1001/jama.1970.03170160047009] [PMID: 4985507] ]. Parenteral nutrition aims to provide nutritional requirements in circumstances where full enteral feeds will be delayed or inadequate [49Scribner BH, Cole JJ, Christopher TG, Vizzo JE, Atkins RC, Blagg CR. Long-term total parenteral nutrition. The concept of an artificial gut. JAMA 1970; 212(3): 457-63.
[http://dx.doi.org/10.1001/jama.1970.03170160047009] [PMID: 4985507] ]. The process calls for an intravenous infusion of macronutrients, micronutrients, and electrolytes. However, PN can cause side effects. The most common side effects include liver dysfunction (cholestasis, steatosis, steatohepatitis and liver cirrhosis) [50Staun M, Pironi L, Bozzetti F, et al. ESPEN guidelines on parenteral nutrition: Home parenteral nutrition (HPN) in adult patients. Clin Nutr 2009; 28(4): 467-79.
[http://dx.doi.org/10.1016/j.clnu.2009.04.001] [PMID: 19464089] ] and loss of bone mineral density [51Pironi L, Tjellesen L, De Francesco A, et al. Bone mineral density in patients on home parenteral nutrition: A follow-up study. Clin Nutr 2004; 23(6): 1288-302.
[http://dx.doi.org/10.1016/j.clnu.2004.04.003] [PMID: 15556251] ]. Selenium deficiency is another finding observed in PN. With the usage of PN as treatments for the increased incidence of SBS/intestinal failure [52M’Koma AE. Inflammatory bowel disease: An expanding global health problem. Clin Med Insights Gastroenterol 2013; 6: 33-47.
[http://dx.doi.org/10.4137/CGast.S12731] [PMID: 24833941] -54Zwintscher NP, Azarow KS, Horton JD, Newton CR, Martin MJ. The increasing incidence of adolescent bariatric surgery. J Pediatr Surg 2013; 48(12): 2401-7.
[http://dx.doi.org/10.1016/j.jpedsurg.2013.08.015] [PMID: 24314178] ]. In this review, we identified 23 cases of selenium-responsive cardiomyopathy due to selenium deficiency secondary to a gastrointestinal etiology (Table 3).
3.3. Selenium Deficiency and Cardiomyopathy in Patients with AIDS without HAART
Heart failure among patients infected with HIV is common [55Hsue PY, Waters DD. Heart failure in persons living with HIV infection. Curr Opin HIV AIDS 2017; 12(6): 534-9.
[http://dx.doi.org/10.1097/COH.0000000000000409] [PMID: 28863015] ]. In the pre-HAART (highly active antiretroviral therapy) era, HIV associated cardiomyopathy was observed in patients with severe AIDS [55Hsue PY, Waters DD. Heart failure in persons living with HIV infection. Curr Opin HIV AIDS 2017; 12(6): 534-9.
[http://dx.doi.org/10.1097/COH.0000000000000409] [PMID: 28863015] ]. Thus, in a study involving 3,000 pediatric AIDS patients, HAART decreased cardiomyopathy from 25.6 cases per 1000 person per year to 3.9 cases [56Patel K, Van Dyke RB, Mittleman MA, Colan SD, Oleske JM, Seage GR III. The impact of HAART on cardiomyopathy among children and adolescents perinatally infected with HIV-1. AIDS 2012; 26(16): 2027-37.
[http://dx.doi.org/10.1097/QAD.0b013e3283578bfa] [PMID: 22781228] ]. It is important to note that most studies investigating decrease in HIV cardiomyopathy due to HAART are conducted in Europe or in America [57Remick J, Georgiopoulou V, Marti C, et al. Heart failure in patients with human immunodeficiency virus infection: Epidemiology, pathophysiology, treatment, and future research. Circulation 2014; 129(17): 1781-9.
[http://dx.doi.org/10.1161/CIRCULATIONAHA.113.004574] [PMID: 24778120] ]. However, AIDS patients in economically impoverished regions, such as the Sub-Saharan Africa [58UNAIDS. 2006 UNAIDS Fact sheet: Global facts and figures.
http:// data.unaids.org/ pub/ globalreport/ 2006/200605-fs_globalfactsfigures_en.pdf], have very limited access to HAART. More specifically, an approximate 32% increase in the prevalence of HIV cardiomyopathy and related mortality was observed in developing nations where HAART access is limited, and thus pathogenic impact of nutritional factors is more significant [59Barbaro G. Reviewing the cardiovascular complications of HIV infection after the introduction of highly active antiretroviral therapy. Curr Drug Targets Cardiovasc Haematol Disord 2005; 5(4): 337-43.
[http://dx.doi.org/10.2174/1568006054553444] [PMID: 16101566] ]. Although most studies of the relationship between serum selenium levels and HIV infection in humans measured the effect of selenium on CD4 cell counts and/or HIV seropositivity, there is some evidence for a relationship between selenium deficiency and development of HIV associated cardiomyopathy. Furthermore, selenium supplementation appears to improve the cardiac function in AIDS patients. For example, a prospective multicenter study of 416 HIV positive patients in Rwanda who had no access to HAART and did not have a documented history of cardiovascular disease revealed a significant association of low serum level of selenium with the development of cardiomyopathy [60Twagirumukiza M, Nkeramihigo E, Seminega B, Gasakure E, Boccara F, Barbaro G. Prevalence of dilated cardiomyopathy in HIV-infected African patients not receiving HAART: A multicenter, observational, prospective, cohort study in Rwanda. Curr HIV Res 2007; 5(1): 129-37.
[http://dx.doi.org/10.2174/157016207779316288] [PMID: 17266564] ]. In this review, we identified three studies of HIV associated selenium-responsive cardiomyopathy that result from selenium deficiency (Table 3). The findings of one particular report by Zazzo et al. [61Zazzo JF, Chalas J, Lafont A, Camus F, Chappuis P. Is nonobstructive cardiomyopathy in AIDS a selenium deficiency-related disease? JPEN J Parenter Enteral Nutr 1988; 12(5): 537-8.
[http://dx.doi.org/10.1177/0148607188012005537] [PMID: 3184430] ] is not included in the Table because certain patients information and lab values (patient gender, age, and serum selenium values) were not reported. Nevertheless, it is important to note that Zazzo et al. reported the effect of selenium treatment of eight AIDS patients with nonobstructive cardiomyopathy. This group of patients were diagnosed according to changes in the value of left ventricular shortening fraction, and low serum selenium. These patients were treated with oral sodium selenite (800 μg/day for 15 days and then 400 μg/day for 8 days). Weekly evaluations showed that the treatment normalized left ventricular function in six patients, one patient died on the 15th day, and one patient also had a thiamine deficiency [61Zazzo JF, Chalas J, Lafont A, Camus F, Chappuis P. Is nonobstructive cardiomyopathy in AIDS a selenium deficiency-related disease? JPEN J Parenter Enteral Nutr 1988; 12(5): 537-8.
[http://dx.doi.org/10.1177/0148607188012005537] [PMID: 3184430] ]. Nevertheless, the response to selenium treatment by the AIDS patients listed in Table 3 and reported by Zazzo et al. demonstrated a causal relationship between selenium deficiency and HIV associated cardiomyopathy.
3.4. Selenium Deficiency and Cardiomyopathy in a Patient with Dystrophic Epidermolysis Bullosa
Recessive dystrophic epidermolysis bullosa (RDEB) is a condition in which the formation of anchoring fibril at the dermal-epidermal junction is disrupted and it is thought to be brought about by mutations in type VII collagen [62Rashidghamat E, McGrath JA. Novel and emerging therapies in the treatment of recessive dystrophic epidermolysis bullosa. Intractable Rare Dis Res 2017; 6(1): 6-20.
[http://dx.doi.org/10.5582/irdr.2017.01005] [PMID: 28357176] ]. Patients with RDEB develop skin blister and skin fragility due to minimal trauma [62Rashidghamat E, McGrath JA. Novel and emerging therapies in the treatment of recessive dystrophic epidermolysis bullosa. Intractable Rare Dis Res 2017; 6(1): 6-20.
[http://dx.doi.org/10.5582/irdr.2017.01005] [PMID: 28357176] ] and are at risk of developing cardiomyopathy if they acquire selenium deficiency [63Rashidghamat E, McGrath JA. Novel and emerging therapies in the treatment of recessive dystrophic epidermolysis bullosa. Intractable Rare Dis Res 2017; 6(1): 6-20.
[http://dx.doi.org/10.5582/irdr.2017.01005] [PMID: 28357176] ]. This review identified one case report of selenium-responsive cardiomyopathy due to selenium deficiency in a RDEB patient (Table 3).
4. DISCUSSION
Keshan disease is a well-documented dilated cardiomyopathy described as focal myocardial necrosis with normal coronary arteries [64]. It was an endemic disease with high incidence in regions where the soil has little selenium [64]. Dietary sources of selenium include seafood, meat and cereals [65Bergqvist AGC, Chee CM, Lutchka L, Rychik J, Stallings VA. Selenium deficiency associated with cardiomyopathy: A complication of the ketogenic diet. Epilepsia 2003; 44(4): 618-20.
[http://dx.doi.org/10.1046/j.1528-1157.2003.26102.x] [PMID: 12681013] ]. This portends that human selenium levels is contingent on the type of food intake and source of cereal – whether the grains for cereal production are grown in selenium rich soil or deficient in selenium [65Bergqvist AGC, Chee CM, Lutchka L, Rychik J, Stallings VA. Selenium deficiency associated with cardiomyopathy: A complication of the ketogenic diet. Epilepsia 2003; 44(4): 618-20.
[http://dx.doi.org/10.1046/j.1528-1157.2003.26102.x] [PMID: 12681013] ]. The ketogenic diet, which consists mostly of fat in addition to some fish/eggs/meats, has insufficient amounts of selenium compared to a more balanced diet [66National Research Council, Subcommittee on the Tenth Edition of the RDAs. Recommended dietary allowances 10th rev. ed.. 1989.]. For example, Bergqvist et al. conducted a retrospective analysis of the ketogenic diet and showed a deficiency in selenium [65Bergqvist AGC, Chee CM, Lutchka L, Rychik J, Stallings VA. Selenium deficiency associated with cardiomyopathy: A complication of the ketogenic diet. Epilepsia 2003; 44(4): 618-20.
[http://dx.doi.org/10.1046/j.1528-1157.2003.26102.x] [PMID: 12681013] ]. In the case report by Bank et al., although they did not report an analysis of their patient’s ketogenic diet, their post mortem cardiomyocyte examination was consistent with findings observed in the context of selenium deficiency [67Bank IM, Shemie SD, Rosenblatt B, Bernard C, Mackie AS. Sudden cardiac death in association with the ketogenic diet. Pediatr Neurol 2008; 39(6): 429-31.
[http://dx.doi.org/10.1016/j.pediatrneurol.2008.08.013] [PMID: 19027591] ]. Similarly, although Sirikonda et al. did not report the ketogenic diet content of their patient, their diagnosis of the patient with selenium deficiency-induced cardiomyopathy was supported by the fact that the patient had low serum selenium level and that selenium supplementation and termination of the ketogenic diet resulted in cardiac improvement [68Sirikonda NS, Patten WD, Phillips JR, Mullett CJ. Ketogenic diet: Rapid onset of selenium deficiency-induced cardiac decompensation. Pediatr Cardiol 2012; 33(5): 834-8.
[http://dx.doi.org/10.1007/s00246-012-0219-6] [PMID: 22367552] ].
Similar to ketogenic diet, PN may also be low in selenium [69Zabel NL, Harland J, Gormican AT, Ganther HE. Selenium content of commercial formula diets. Am J Clin Nutr 1978; 31(5): 850-8.
[http://dx.doi.org/10.1093/ajcn/31.5.850] [PMID: 417618] ]. The amount of selenium provided in PN is typically 20-60 μg/day, which is inadequate [70Vanek VW, Borum P, Buchman A, et al. A.S.P.E.N. position paper: Recommendations for changes in commercially available parenteral multivitamin and multi-trace element products. Nutr Clin Pract 2012; 27(4): 440-91.
[http://dx.doi.org/10.1177/0884533612446706] [PMID: 22730042] , 71Btaiche IF, Carver PL, Welch KB. Dosing and monitoring of trace elements in long-term home parenteral nutrition patients. JPEN J Parenter Enteral Nutr 2011; 35(6): 736-47.
[http://dx.doi.org/10.1177/0148607111413902] [PMID: 21825087] ]. An increase of selenium in PN has been proposed to 60-100 μg/day [70Vanek VW, Borum P, Buchman A, et al. A.S.P.E.N. position paper: Recommendations for changes in commercially available parenteral multivitamin and multi-trace element products. Nutr Clin Pract 2012; 27(4): 440-91.
[http://dx.doi.org/10.1177/0884533612446706] [PMID: 22730042] , 71Btaiche IF, Carver PL, Welch KB. Dosing and monitoring of trace elements in long-term home parenteral nutrition patients. JPEN J Parenter Enteral Nutr 2011; 35(6): 736-47.
[http://dx.doi.org/10.1177/0148607111413902] [PMID: 21825087] ], given selenium requirements are increased in malabsorptive states [71Btaiche IF, Carver PL, Welch KB. Dosing and monitoring of trace elements in long-term home parenteral nutrition patients. JPEN J Parenter Enteral Nutr 2011; 35(6): 736-47.
[http://dx.doi.org/10.1177/0148607111413902] [PMID: 21825087] ]. Regardless of this adjustment, higher doses of selenium in parenteral nutrition might still be warranted for patients with SBS and non-SBS [71Btaiche IF, Carver PL, Welch KB. Dosing and monitoring of trace elements in long-term home parenteral nutrition patients. JPEN J Parenter Enteral Nutr 2011; 35(6): 736-47.
[http://dx.doi.org/10.1177/0148607111413902] [PMID: 21825087] ]. In a study conducted by Milan et al., 68 patients receiving long term care for SBS using parenteral nutrition had significant decline in serum selenium levels [72Dastych M Jr, Šenkyřík M, Dastych M, et al. Trace element status (Zinc, Copper, Selenium, Iron, Manganese) in patients with long-term home parenteral nutrition. Ann Nutr Metab 2016; 69(2): 120-4.
[http://dx.doi.org/10.1159/000450763] [PMID: 27736814] ]. On the contrary, other trace elements, such as zinc, copper and iron, had no significant difference between patients with PN and control subjects [72Dastych M Jr, Šenkyřík M, Dastych M, et al. Trace element status (Zinc, Copper, Selenium, Iron, Manganese) in patients with long-term home parenteral nutrition. Ann Nutr Metab 2016; 69(2): 120-4.
[http://dx.doi.org/10.1159/000450763] [PMID: 27736814] ], suggesting that the selenium provided by the conventional PN is insufficient and explaining why selenium deficiency was observed in a significant number of patients on PN. The cases we reviewed involved patients with lower nutritional status stemming from various etiologies such as lack or reduced selenium in their oral/parenteral diet and numerous gastrointestinal pathologies that resulted in intestinal failure. Given that sick patients tend to have a poor appetite and hence lower nutritional status, poor selenium intake seems an important determinant for the selenium deficiency of the patients in Tables 2 and 3. Thus, it is important to make certain that PN is supplemented with sufficient selenium and that the selenium levels of patients need to be continuously monitored to prevent the development of selenium deficiency related conditions including, but not limited to, cardiomyopathy.
Patients suffering from AIDS are another group of patients who are at risk of selenium deficiency. Some AIDS patients developed HIV associated cardiac abnormalities similar to those found in Keshan disease [73Dworkin BM, Antonecchia PP, Smith F, et al. Reduced cardiac selenium content in the acquired immunodeficiency syndrome. JPEN J Parenter Enteral Nutr 1989; 13(6): 644-7.
[http://dx.doi.org/10.1177/0148607189013006644] [PMID: 2614866] ]. HIV causes impaired systolic function through proinflammatory cytokines such as tumor necrosis factor and IL-1β [55Hsue PY, Waters DD. Heart failure in persons living with HIV infection. Curr Opin HIV AIDS 2017; 12(6): 534-9.
[http://dx.doi.org/10.1097/COH.0000000000000409] [PMID: 28863015] ]. In conjunction with these findings, there are reports of sudden cardiac death in selenium deficiency patients triggered by sepsis [74Lockitch G, Taylor GP, Wong LT, et al. Cardiomyopathy associated with nonendemic selenium deficiency in a Caucasian adolescent. Am J Clin Nutr 1990; 52(3): 572-7.
[http://dx.doi.org/10.1093/ajcn/52.3.572] [PMID: 2168125] ]. However, the pathophysiology of how infections alters the immune systems response in selenium deficient patients leading to cardiomyopathy remains elusive. The change in the immune systems reaction may underlie the susceptibility of cardiomyopathies of individuals with inadequate selenium nutrition.
We have identified two cases of cardiomyopathy due to selenium deficiency associated with RDEB reported by Melville et al. [75Melville C, Atherton D, Burch M, Cohn A, Sullivan I. Fatal cardiomyopathy in dystrophic epidermolysis bullosa. Br J Dermatol 1996; 135(4): 603-6.
[http://dx.doi.org/10.1111/j.1365-2133.1996.tb03840.x] [PMID: 8915155] ]. Although, according to the report, the patients involved were malnourished, numerous patients with RDEB (14 to 25 patients) had reduced serum selenium levels without cardiomyopathy [75Melville C, Atherton D, Burch M, Cohn A, Sullivan I. Fatal cardiomyopathy in dystrophic epidermolysis bullosa. Br J Dermatol 1996; 135(4): 603-6.
[http://dx.doi.org/10.1111/j.1365-2133.1996.tb03840.x] [PMID: 8915155] ]. Thus, it is possible that patients with RDBE are at risk of developing selenium deficiency, which, if worsens, can lead to cardiomyopathy. Thus, Melville et al. recommended that patients with severe RDEB should be examined carefully for their nutritional intake including selenium and with regular echocardiographic screening for signs of cardiomyopathy.
It is important to note that the selenium status of the patients in Tables 2 and 3 was determined by serum selenium levels, and that majority of the cases involved sick patients who might have had other unknown confounding factors that cause selenium deficiency. An alternative method of assessing selenium status is measuring intracellular concentrations or the activities of selenoproteins [5Arnér ESJ, Holmgren A. Physiological functions of thioredoxin and thioredoxin reductase. Eur J Biochem 2000; 267(20): 6102-9.
[http://dx.doi.org/10.1046/j.1432-1327.2000.01701.x] [PMID: 11012661] , 27Guo Z, Van Remmen H, Yang H, et al. Changes in expression of antioxidant enzymes affect cell-mediated LDL oxidation and oxidized LDL-induced apoptosis in mouse aortic cells. Arterioscler Thromb Vasc Biol 2001; 21(7): 1131-8.
[http://dx.doi.org/10.1161/hq0701.092092] [PMID: 11451741] , 33Wu Q, Huang K. Effect of selenium compounds on the damage induced by oxysterol on rat arterial walls. Biol Trace Elem Res 2006; 112(3): 273-82.
[http://dx.doi.org/10.1385/BTER:112:3:273] [PMID: 17057266] ]. Ideally, the selenium status should be monitored by multiple methods that determine not only the serum level but also intracellular level and the activities of selenoproteins, such as glutathione peroxidase in cells. Nevertheless, the fact that the cardiomyopathy symptoms were alleviated after administration of selenium in most patients reviewed suggests that the measurement of serum selenium level is a useful way to determine selenium status of patients with cardiomyopathy symptoms.
The cases of cardiovascular dysfunction listed in Table 2 and 3 represent selenium-responsive cases and furthermore show distinctive patient populations who are at risk of developing cardiovascular dysfunction due to selenium deficiency. However, it is possible that selenium deficiency in populations of patients with these diseases and/or other diseases is underreported because those patients who have yet to present with cardiovascular dysfunction are unlikely to receive selenium status analysis. Therefore, it is important that physicians think about including selenium status analysis for patients with certain diseases (IBS, AIDS, and RDBE, for example) and/or receiving certain treatment, such as ketogenic diet and parenteral nutrition. Doing so is important because physicians can take simple management – selenium supplementation – to prevent the development of cardiovascular dysfunctions, such as cardiomyopathy, in these patients.
CONCLUSION
These cases in the literature emphasize the need for considering selenium deficiency as a potential cause of the development of cardiomyopathy in patients in various diseases states and treatments. We hope to draw attention to implement preventative measures of selenium deficiency-induced cardiomyopathy in these clinical contexts. Moreover, we hope to spark more investigation into the pathophysiology of selenium induced cardiomyopathy.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise
ACKNOWLEDGEMENTS
Nigel Amankwah: Performed research, collected data, organized and analyzed data, wrote paper
Zhiyong Han: Designed research, analyzed data, revised manuscript
REFERENCES
| [1] | Small-Howard AL, Berry MJ. Unique features of selenocysteine incorporation function within the context of general eukaryotic translational processes. Biochemical Society Transactions Portland Press Limited 2005; 33(): 1493-7. |
| [2] | Berry MJ, Tujebajeva RM, Copeland PR, et al. Selenocysteine incorporation directed from the 3'UTR: Characterization of eukaryotic EFsec and mechanistic implications. Biofactors 2001; 14(1-4): 17-24. [http://dx.doi.org/10.1002/biof.5520140104] [PMID: 11568436] |
| [3] | Bellinger FP, Raman AV, Reeves MA, Berry MJ. Regulation and function of selenoproteins in human disease. Biochem J 2009; 422(1): 11-22. [http://dx.doi.org/10.1042/BJ20090219] [PMID: 19627257] |
| [4] | Forstrom JW, Zakowski JJ, Tappel AL. Identification of the catalytic site of rat liver glutathione peroxidase as selenocysteine. Biochem 1978; 17(13): 2639-44. [http://dx.doi.org/10.1021/bi00606a028] [PMID: 678534] |
| [5] | Arnér ESJ, Holmgren A. Physiological functions of thioredoxin and thioredoxin reductase. Eur J Biochem 2000; 267(20): 6102-9. [http://dx.doi.org/10.1046/j.1432-1327.2000.01701.x] [PMID: 11012661] |
| [6] | Moskovitz J. Methionine sulfoxide reductases: Ubiquitous enzymes involved in antioxidant defense, protein regulation, and prevention of aging-associated diseases. Biochim Biophys Acta 2005; 1703(2): 213-9. [http://dx.doi.org/10.1016/j.bbapap.2004.09.003] [PMID: 15680229] |
| [7] | Bianco AC, Kim BW. Deiodinases: Implications of the local control of thyroid hormone action. J Clin Invest 2006; 116(10): 2571-9. [http://dx.doi.org/10.1172/JCI29812] [PMID: 17016550] |
| [8] | Schomburg L, Schweizer U, Holtmann B, Flohé L, Sendtner M, Köhrle J. Gene disruption discloses role of selenoprotein P in selenium delivery to target tissues. Biochem J 2003; 370(Pt 2): 397-402. [http://dx.doi.org/10.1042/bj20021853] [PMID: 12521380] |
| [9] | Hwang DY, Cho JS, Oh JH, et al. Differentially expressed genes in transgenic mice carrying human mutant presenilin-2 (N141I): Correlation of selenoprotein M with Alzheimer’s disease. Neurochem Res 2005; 30(8): 1009-19. [http://dx.doi.org/10.1007/s11064-005-6787-6] [PMID: 16258850] |
| [10] | Zorzato F, Jungbluth H, Zhou H, Muntoni F, Treves S. Functional effects of mutations identified in patients with multiminicore disease. IUBMB Life 2007; 59(1): 14-20. [http://dx.doi.org/10.1080/15216540601187803] [PMID: 17365175] |
| [11] | Grumolato L, Ghzili H, Montero-Hadjadje M, et al. Selenoprotein T is a PACAP-regulated gene involved in intracellular Ca2+ mobilization and neuroendocrine secretion. FASEB J 2008; 22(6): 1756-68. [http://dx.doi.org/10.1096/fj.06-075820] [PMID: 18198219] |
| [12] | Ye Y, Shibata Y, Yun C, Ron D, Rapoport TA. A membrane protein complex mediates retro-translocation from the ER lumen into the cytosol. Nature 2004; 429(6994): 841-7. [http://dx.doi.org/10.1038/nature02656] [PMID: 15215856] |
| [13] | Ashrafi MR, Shabanian R, Abbaskhanian A, et al. Selenium and intractable epilepsy: Is there any correlation? Pediatr Neurol 2007; 36(1): 25-9. [http://dx.doi.org/10.1016/j.pediatrneurol.2006.09.001] [PMID: 17162193] |
| [14] | Savaskan NE, Bräuer AU, Kühbacher M, et al. Selenium deficiency increases susceptibility to glutamate-induced excitotoxicity. FASEB J 2003; 17(1): 112-4. [http://dx.doi.org/10.1096/fj.02-0067fje] [PMID: 12424220] |
| [15] | Hill KE, Zhou J, McMahan WJ, et al. Deletion of selenoprotein P alters distribution of selenium in the mouse. J Biol Chem 2003; 278(16): 13640-6. [http://dx.doi.org/10.1074/jbc.M300755200] [PMID: 12574155] |
| [16] | Jungbluth H. Multi-minicore disease. Orphanet J Rare Dis 2007; 2: 31. [http://dx.doi.org/10.1186/1750-1172-2-31] [PMID: 17631035] |
| [17] | Zalk R, Lehnart SE, Marks AR. Modulation of the ryanodine receptor and intracellular calcium. Annu Rev Biochem 2007; 76: 367-85. [http://dx.doi.org/10.1146/annurev.biochem.76.053105.094237] [PMID: 17506640] |
| [18] | Jurynec MJ, Xia R, Mackrill JJ, et al. Selenoprotein N is required for ryanodine receptor calcium release channel activity in human and zebrafish muscle. Proc Natl Acad Sci USA 2008; 105(34): 12485-90. [http://dx.doi.org/10.1073/pnas.0806015105] [PMID: 18713863] |
| [19] | Deniziak M, Thisse C, Rederstorff M, Hindelang C, Thisse B, Lescure A. Loss of selenoprotein N function causes disruption of muscle architecture in the zebrafish embryo. Exp Cell Res 2007; 313(1): 156-67. [http://dx.doi.org/10.1016/j.yexcr.2006.10.005] [PMID: 17123513] |
| [20] | Reeves MA, Bellinger FP, Berry MJ. The neuroprotective functions of selenoprotein M and its role in cytosolic calcium regulation. Antioxid Redox Signal 2010; 12(7): 809-18. [http://dx.doi.org/10.1089/ars.2009.2883] [PMID: 19769485] |
| [21] | Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. N Engl J Med 2001; 344(7): 501-9. [http://dx.doi.org/10.1056/NEJM200102153440707] [PMID: 11172193] |
| [22] | Lum H, Roebuck KA. Oxidant stress and endothelial cell dysfunction. Am J Physiol Cell Physiol 2001; 280(4): C719-41. [http://dx.doi.org/10.1152/ajpcell.2001.280.4.C719] [PMID: 11245588] |
| [23] | Arthur JR. The glutathione peroxidases. Cell Mol Life Sci 2000; 57(13-14): 1825-35. [PMID: 11215509] |
| [24] | Maulik N, Yoshida T, Das DK. Regulation of cardiomyocyte apoptosis in ischemic reperfused mouse heart by glutathione peroxidase. Mol Cell Biochem 1999; 196(1-2): 13-21. [http://dx.doi.org/10.1023/A:1006905910140] [PMID: 10448898] |
| [25] | Forgione MA, Cap A, Liao R, et al. Heterozygous cellular glutathione peroxidase deficiency in the mouse: Abnormalities in vascular and cardiac function and structure. Circulation 2002; 106(9): 1154-8. [http://dx.doi.org/10.1161/01.CIR.0000026820.87824.6A] [PMID: 12196344] |
| [26] | Kenet G, Freedman J, Shenkman B, et al. Plasma glutathione peroxidase deficiency and platelet insensitivity to nitric oxide in children with familial stroke. Arterioscler Thromb Vasc Biol 1999; 19(8): 2017-23. [http://dx.doi.org/10.1161/01.ATV.19.8.2017] [PMID: 10446087] |
| [27] | Guo Z, Van Remmen H, Yang H, et al. Changes in expression of antioxidant enzymes affect cell-mediated LDL oxidation and oxidized LDL-induced apoptosis in mouse aortic cells. Arterioscler Thromb Vasc Biol 2001; 21(7): 1131-8. [http://dx.doi.org/10.1161/hq0701.092092] [PMID: 11451741] |
| [28] | Miller S, Walker SW, Arthur JR, et al. Selenite protects human endothelial cells from oxidative damage and induces thioredoxin reductase. Clin Sci (Lond) 2001; 100(5): 543-50. [http://dx.doi.org/10.1042/cs1000543] [PMID: 11294695] |
| [29] | Steinbrenner H, Alili L, Bilgic E, Sies H, Brenneisen P. Involvement of selenoprotein P in protection of human astrocytes from oxidative damage. Free Radic Biol Med 2006; 40(9): 1513-23. [http://dx.doi.org/10.1016/j.freeradbiomed.2005.12.022] [PMID: 16632112] |
| [30] | Tang R, Liu H, Wang T, Huang K. Mechanisms of selenium inhibition of cell apoptosis induced by oxysterols in rat vascular smooth muscle cells. Arch Biochem Biophys 2005; 441(1): 16-24. [http://dx.doi.org/10.1016/j.abb.2005.06.006] [PMID: 16039982] |
| [31] | Thomas JP, Geiger PG, Girotti AW. Lethal damage to endothelial cells by oxidized low density lipoprotein: Role of selenoperoxidases in cytoprotection against lipid hydroperoxide- and iron-mediated reactions. J Lipid Res 1993; 34(3): 479-90. [PMID: 8468531] |
| [32] | Huang K, Liu H, Chen Z, Xu H. Role of selenium in cytoprotection against cholesterol oxide-induced vascular damage in rats. Atherosclerosis 2002; 162(1): 137-44. [http://dx.doi.org/10.1016/S0021-9150(01)00707-9] [PMID: 11947907] |
| [33] | Wu Q, Huang K. Effect of selenium compounds on the damage induced by oxysterol on rat arterial walls. Biol Trace Elem Res 2006; 112(3): 273-82. [http://dx.doi.org/10.1385/BTER:112:3:273] [PMID: 17057266] |
| [34] | Conrad M, Jakupoglu C, Moreno SG, et al. Essential role for mitochondrial thioredoxin reductase in hematopoiesis, heart development, and heart function. Mol Cell Biol 2004; 24(21): 9414-23. [http://dx.doi.org/10.1128/MCB.24.21.9414-9423.2004] [PMID: 15485910] |
| [35] | Horstkotte J, Perisic T, Schneider M, et al. Mitochondrial thioredoxin reductase is essential for early postischemic myocardial protection. Circulation 2011; 124(25): 2892-902. [http://dx.doi.org/10.1161/CIRCULATIONAHA.111.059253] [PMID: 22144571] |
| [36] | Sibbing D, Pfeufer A, Perisic T, et al. Mutations in the mitochondrial thioredoxin reductase gene TXNRD2 cause dilated cardiomyopathy. Eur Heart J 2011; 32(9): 1121-33. [http://dx.doi.org/10.1093/eurheartj/ehq507] [PMID: 21247928] |
| [37] | Chen J. An original discovery: Selenium deficiency and Keshan disease (an endemic heart disease). Asia Pac J Clin Nutr 2012; 21(3): 320-6. [PMID: 22705420] |
| [38] | Salonen JT, Alfthan G, Huttunen JK, Pikkarainen J, Puska P. Association between cardiovascular death and myocardial infarction and serum selenium in a matched-pair longitudinal study. Lancet 1982; 2(8291): 175-9. [http://dx.doi.org/10.1016/S0140-6736(82)91028-5] [PMID: 6123886] |
| [39] | Levander OA, Beck MA. Interacting nutritional and infectious etiologies of Keshan disease. Insights from coxsackie virus B-induced myocarditis in mice deficient in selenium or vitamin E. Biol Trace Elem Res 1997; 56(1): 5-21. [http://dx.doi.org/10.1007/BF02778980] [PMID: 9152508] |
| [40] | Beck MA. Nutritionally induced oxidative stress: Effect on viral disease. Am J Clin Nutr 2000; 71(6)(Suppl.): 1676S-81S. [http://dx.doi.org/10.1093/ajcn/71.6.1676S] [PMID: 10837315] |
| [41] | Levy RG, Cooper PN, Giri P. Ketogenic diet and other dietary treatments for epilepsy. Cochrane Database Syst Rev 2012; 3(3): CD001903. [PMID: 22419282] |
| [42] | Perucca P, Scheffer IE, Kiley M. The management of epilepsy in children and adults. Med J Aust 2018; 208(5): 226-33. [http://dx.doi.org/10.5694/mja17.00951] [PMID: 29540143] |
| [43] | Winesett SP, Bessone SK, Kossoff EH. The ketogenic diet in pharmacoresistant childhood epilepsy. Expert Rev Neurother 2015; 15(6): 621-8. [http://dx.doi.org/10.1586/14737175.2015.1044982] [PMID: 25994046] |
| [44] | Seo JH, Lee YM, Lee JS, Kang HC, Kim HD. Efficacy and tolerability of the ketogenic diet according to lipid:nonlipid ratios: Comparison of 3:1 with 4:1 diet. Epilepsia 2007; 48(4): 801-5. [http://dx.doi.org/10.1111/j.1528-1167.2007.01025.x] [PMID: 17386059] |
| [45] | Azevedo de Lima P, Baldini Prudêncio M, Murakami DK, Pereira de Brito Sampaio L, Figueiredo Neto AM, Teixeira Damasceno NR. Effect of classic ketogenic diet treatment on lipoprotein subfractions in children and adolescents with refractory epilepsy. Nutrition 2017; 33: 271-7. [http://dx.doi.org/10.1016/j.nut.2016.06.016] [PMID: 27712963] |
| [46] | Lin A, Turner Z, Doerrer SC, Stanfield A, Kossoff EH. Complications during ketogenic diet initiation: Prevalence, Treatment, and influence on seizure outcomes. Pediatr Neurol 2017; 68: 35-9. [http://dx.doi.org/10.1016/j.pediatrneurol.2017.01.007] [PMID: 28188074] |
| [47] | Patel KS, Carroll R. Hormonal management of small bowel failure. Clin Transl Gastroenterol 2017; 8(6): e105. [http://dx.doi.org/10.1038/ctg.2017.32] [PMID: 28662020] |
| [48] | Wanten G, Calder PC, Forbes A. Managing adult patients who need home parenteral nutrition. BMJ 2011; 342: d1447. [http://dx.doi.org/10.1136/bmj.d1447] [PMID: 21421667] |
| [49] | Scribner BH, Cole JJ, Christopher TG, Vizzo JE, Atkins RC, Blagg CR. Long-term total parenteral nutrition. The concept of an artificial gut. JAMA 1970; 212(3): 457-63. [http://dx.doi.org/10.1001/jama.1970.03170160047009] [PMID: 4985507] |
| [50] | Staun M, Pironi L, Bozzetti F, et al. ESPEN guidelines on parenteral nutrition: Home parenteral nutrition (HPN) in adult patients. Clin Nutr 2009; 28(4): 467-79. [http://dx.doi.org/10.1016/j.clnu.2009.04.001] [PMID: 19464089] |
| [51] | Pironi L, Tjellesen L, De Francesco A, et al. Bone mineral density in patients on home parenteral nutrition: A follow-up study. Clin Nutr 2004; 23(6): 1288-302. [http://dx.doi.org/10.1016/j.clnu.2004.04.003] [PMID: 15556251] |
| [52] | M’Koma AE. Inflammatory bowel disease: An expanding global health problem. Clin Med Insights Gastroenterol 2013; 6: 33-47. [http://dx.doi.org/10.4137/CGast.S12731] [PMID: 24833941] |
| [53] | Peery AF, Dellon ES, Lund J, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology 2012; 143(5): 1179-1187.e3. [http://dx.doi.org/10.1053/j.gastro.2012.08.002] [PMID: 22885331] |
| [54] | Zwintscher NP, Azarow KS, Horton JD, Newton CR, Martin MJ. The increasing incidence of adolescent bariatric surgery. J Pediatr Surg 2013; 48(12): 2401-7. [http://dx.doi.org/10.1016/j.jpedsurg.2013.08.015] [PMID: 24314178] |
| [55] | Hsue PY, Waters DD. Heart failure in persons living with HIV infection. Curr Opin HIV AIDS 2017; 12(6): 534-9. [http://dx.doi.org/10.1097/COH.0000000000000409] [PMID: 28863015] |
| [56] | Patel K, Van Dyke RB, Mittleman MA, Colan SD, Oleske JM, Seage GR III. The impact of HAART on cardiomyopathy among children and adolescents perinatally infected with HIV-1. AIDS 2012; 26(16): 2027-37. [http://dx.doi.org/10.1097/QAD.0b013e3283578bfa] [PMID: 22781228] |
| [57] | Remick J, Georgiopoulou V, Marti C, et al. Heart failure in patients with human immunodeficiency virus infection: Epidemiology, pathophysiology, treatment, and future research. Circulation 2014; 129(17): 1781-9. [http://dx.doi.org/10.1161/CIRCULATIONAHA.113.004574] [PMID: 24778120] |
| [58] | UNAIDS. 2006 UNAIDS Fact sheet: Global facts and figures. http:// data.unaids.org/ pub/ globalreport/ 2006/200605-fs_globalfactsfigures_en.pdf |
| [59] | Barbaro G. Reviewing the cardiovascular complications of HIV infection after the introduction of highly active antiretroviral therapy. Curr Drug Targets Cardiovasc Haematol Disord 2005; 5(4): 337-43. [http://dx.doi.org/10.2174/1568006054553444] [PMID: 16101566] |
| [60] | Twagirumukiza M, Nkeramihigo E, Seminega B, Gasakure E, Boccara F, Barbaro G. Prevalence of dilated cardiomyopathy in HIV-infected African patients not receiving HAART: A multicenter, observational, prospective, cohort study in Rwanda. Curr HIV Res 2007; 5(1): 129-37. [http://dx.doi.org/10.2174/157016207779316288] [PMID: 17266564] |
| [61] | Zazzo JF, Chalas J, Lafont A, Camus F, Chappuis P. Is nonobstructive cardiomyopathy in AIDS a selenium deficiency-related disease? JPEN J Parenter Enteral Nutr 1988; 12(5): 537-8. [http://dx.doi.org/10.1177/0148607188012005537] [PMID: 3184430] |
| [62] | Rashidghamat E, McGrath JA. Novel and emerging therapies in the treatment of recessive dystrophic epidermolysis bullosa. Intractable Rare Dis Res 2017; 6(1): 6-20. [http://dx.doi.org/10.5582/irdr.2017.01005] [PMID: 28357176] |
| [63] | Rashidghamat E, McGrath JA. Novel and emerging therapies in the treatment of recessive dystrophic epidermolysis bullosa. Intractable Rare Dis Res 2017; 6(1): 6-20. [http://dx.doi.org/10.5582/irdr.2017.01005] [PMID: 28357176] |
| [64] | Yusuf SW, Rehman Q, Casscells W. Cardiomyopathy in association with selenium deficiency: A case report. JPEN J Parenter Enteral Nutr 2002; 26(1): 63-6. [http://dx.doi.org/10.1177/014860710202600163] [PMID: 11833754] |
| [65] | Bergqvist AGC, Chee CM, Lutchka L, Rychik J, Stallings VA. Selenium deficiency associated with cardiomyopathy: A complication of the ketogenic diet. Epilepsia 2003; 44(4): 618-20. [http://dx.doi.org/10.1046/j.1528-1157.2003.26102.x] [PMID: 12681013] |
| [66] | National Research Council, Subcommittee on the Tenth Edition of the RDAs. Recommended dietary allowances 10th rev. ed.. 1989. |
| [67] | Bank IM, Shemie SD, Rosenblatt B, Bernard C, Mackie AS. Sudden cardiac death in association with the ketogenic diet. Pediatr Neurol 2008; 39(6): 429-31. [http://dx.doi.org/10.1016/j.pediatrneurol.2008.08.013] [PMID: 19027591] |
| [68] | Sirikonda NS, Patten WD, Phillips JR, Mullett CJ. Ketogenic diet: Rapid onset of selenium deficiency-induced cardiac decompensation. Pediatr Cardiol 2012; 33(5): 834-8. [http://dx.doi.org/10.1007/s00246-012-0219-6] [PMID: 22367552] |
| [69] | Zabel NL, Harland J, Gormican AT, Ganther HE. Selenium content of commercial formula diets. Am J Clin Nutr 1978; 31(5): 850-8. [http://dx.doi.org/10.1093/ajcn/31.5.850] [PMID: 417618] |
| [70] | Vanek VW, Borum P, Buchman A, et al. A.S.P.E.N. position paper: Recommendations for changes in commercially available parenteral multivitamin and multi-trace element products. Nutr Clin Pract 2012; 27(4): 440-91. [http://dx.doi.org/10.1177/0884533612446706] [PMID: 22730042] |
| [71] | Btaiche IF, Carver PL, Welch KB. Dosing and monitoring of trace elements in long-term home parenteral nutrition patients. JPEN J Parenter Enteral Nutr 2011; 35(6): 736-47. [http://dx.doi.org/10.1177/0148607111413902] [PMID: 21825087] |
| [72] | Dastych M Jr, Šenkyřík M, Dastych M, et al. Trace element status (Zinc, Copper, Selenium, Iron, Manganese) in patients with long-term home parenteral nutrition. Ann Nutr Metab 2016; 69(2): 120-4. [http://dx.doi.org/10.1159/000450763] [PMID: 27736814] |
| [73] | Dworkin BM, Antonecchia PP, Smith F, et al. Reduced cardiac selenium content in the acquired immunodeficiency syndrome. JPEN J Parenter Enteral Nutr 1989; 13(6): 644-7. [http://dx.doi.org/10.1177/0148607189013006644] [PMID: 2614866] |
| [74] | Lockitch G, Taylor GP, Wong LT, et al. Cardiomyopathy associated with nonendemic selenium deficiency in a Caucasian adolescent. Am J Clin Nutr 1990; 52(3): 572-7. [http://dx.doi.org/10.1093/ajcn/52.3.572] [PMID: 2168125] |
| [75] | Melville C, Atherton D, Burch M, Cohn A, Sullivan I. Fatal cardiomyopathy in dystrophic epidermolysis bullosa. Br J Dermatol 1996; 135(4): 603-6. [http://dx.doi.org/10.1111/j.1365-2133.1996.tb03840.x] [PMID: 8915155] |
| [76] | Yoshida T, Maulik N, Engelman RM, et al. Glutathione peroxidase knockout mice are susceptible to myocardial ischemia reperfusion injury. Circulation 1997; 96(9)(Suppl.): II-216-20. [PMID: 9386101] |
| [77] | Nan C, Li Y, Jean-Charles P-Y, et al. Deficiency of methionine sulfoxide reductase A causes cellular dysfunction and mitochondrial damage in cardiac myocytes under physical and oxidative stresses. Biochem Biophys Res Commun 2010; 402(4): 608-13. [http://dx.doi.org/10.1016/j.bbrc.2010.10.064] [PMID: 20971073] |
| [78] | Ueta CB, Oskouei BN, Olivares EL, et al. Absence of myocardial thyroid hormone inactivating deiodinase results in restrictive cardiomyopathy in mice. Mol Endocrinol 2012; 26(5): 809-18. [http://dx.doi.org/10.1210/me.2011-1325] [PMID: 22403173] |
| [79] | Kanekura T, Yotsumoto S, Maeno N, et al. Selenium deficiency: Report of a case. Clin Exp Dermatol 2005; 30(4): 346-8. [http://dx.doi.org/10.1111/j.1365-2230.2005.01746.x] [PMID: 15953064] |
| [80] | Inoko M, Konishi T, Matsusue S, Kobashi Y. Midmural fibrosis of left ventricle due to selenium deficiency. Circulation 1998; 98(23): 2638-9. [http://dx.doi.org/10.1161/01.CIR.98.23.2638] [PMID: 9843475] |
| [81] | Levy JB, Jones HW, Gordon AC. Selenium deficiency, reversible cardiomyopathy and short-term intravenous feeding. Postgrad Med J 1994; 70(821): 235-6. [http://dx.doi.org/10.1136/pgmj.70.821.235] [PMID: 8183763] |
| [82] | Sriram K, Peterson JK, O’Gara J, Hammond JM. Clinical improvement of congestive heart failure after selenium supplementation in total parenteral nutrition. Acta Pharmacol Toxicol 1986; 59: 361-4. [http://dx.doi.org/10.1111/j.1600-0773.1986.tb02780.x] [PMID: 3096074] |
| [83] | Johnson RA, Baker SS, Fallon JT, et al. An occidental case of cardiomyopathy and selenium deficiency. N Engl J Med 1981; 304(20): 1210-2. [http://dx.doi.org/10.1056/NEJM198105143042005] [PMID: 6783905] |
| [84] | Marcus RW. Myopathy and cardiomyopathy associated with selenium deficiency: Case report, literature review, and hypothesis. Md Med J 1993; 42(7): 669-74. [PMID: 8412527] |
| [85] | Fleming CR, Lie JT, McCall JT, O’Brien JF, Baillie EE, Thistle JL. Selenium deficiency and fatal cardiomyopathy in a patient on home parenteral nutrition. Gastroenterology 1982; 83(3): 689-93. [PMID: 6807740] |
| [86] | Quercia RA, Korn S, O’Neill D, et al. Selenium deficiency and fatal cardiomyopathy in a patient receiving long-term home parenteral nutrition. Clin Pharm 1984; 3(5): 531-5. [PMID: 6435941] |
| [87] | Munguti CM, Al Rifai M, Shaheen W. A rare cause of cardiomyopathy: A case of selenium deficiency causing severe cardiomyopathy that improved on supplementation. Cureus 2017; 9(8): e1627. [PMID: 29098137] |
| [88] | Boldery R, Fielding G, Rafter T, Pascoe AL, Scalia GM. Nutritional deficiency of selenium secondary to weight loss (bariatric) surgery associated with life-threatening cardiomyopathy. Heart Lung Circ 2007; 16(2): 123-6. [http://dx.doi.org/10.1016/j.hlc.2006.07.013] [PMID: 17324623] |
| [89] | de Berranger E, Colinet S, Michaud L, et al. Severe selenium deficiency secondary to chylous loss. JPEN J Parenter Enteral Nutr 2006; 30(2): 173-4. [http://dx.doi.org/10.1177/0148607106030002173] [PMID: 16517962] |
| [90] | Collipp PJ, Chen SY. Cardiomyopathy and selenium deficiency in a two-year-old girl. N Engl J Med 1981; 304(21): 1304-5. [http://dx.doi.org/10.1056/NEJM198105213042120] [PMID: 7219482] |
| [91] | Al-Matary A, Hussain M, Ali J. Selenium: A brief review and a case report of selenium responsive cardiomyopathy. BMC Pediatr 2013; 13: 39. [http://dx.doi.org/10.1186/1471-2431-13-39] [PMID: 23530936] |
| [92] | Saliba W, El Fakih R, Shaheen W. Heart failure secondary to selenium deficiency, reversible after supplementation. Int J Cardiol 2010; 141(2): e26-7. [http://dx.doi.org/10.1016/j.ijcard.2008.11.095] [PMID: 19059654] |
| [93] | Reeves WC, Marcuard SP, Willis SE, Movahed A. Reversible cardiomyopathy due to selenium deficiency. JPEN J Parenter Enteral Nutr 1989; 13(6): 663-5. [http://dx.doi.org/10.1177/0148607189013006663] [PMID: 2614867] |
| [94] | Briel A, Veber B, Dureuil B. Selenium deficiency favors the appearance of heart failure after multiple injury. Ann Fr Anesth Reanim 1997; 16(7): 911-2. [http://dx.doi.org/10.1016/S0750-7658(97)89841-3] [PMID: 9750622] |
| [95] | Burke MP, Opeskin K. Fulminant heart failure due to selenium deficiency cardiomyopathy (Keshan disease). Med Sci Law 2002; 42(1): 10-3. [http://dx.doi.org/10.1177/002580240204200103] [PMID: 11848134] |
| [96] | Harris S, Naina HVK, Beckman TJ. Heartbreaking weight loss. Am J Gastroenterol 2009; 104(5): 1322-3. [http://dx.doi.org/10.1038/ajg.2009.37] [PMID: 19319124] |
| [97] | Massoure P-L, Camus O, Fourcade L, Simon F. Bilateral leg oedema after bariatric surgery: A selenium-deficient cardiomyopathy. Obes Res Clin Pract 2017; 11(5): 622-6. [http://dx.doi.org/10.1016/j.orcp.2017.05.004] [PMID: 28610944] |
| [98] | Constans J, Sire S, Sergeant C, Simonoff M, Ragnaud JM. Dilated cardiomyopathy and selenium deficiency in AIDS. Apropos of a case. Rev Med Interne 1997; 18(8): 642-5. [http://dx.doi.org/10.1016/S0248-8663(97)82466-6] [PMID: 9365739] |
| [99] | Kavanaugh-McHugh AL, Ruff A, Perlman E, Hutton N, Modlin J, Rowe S. Selenium deficiency and cardiomyopathy in acquired immunodeficiency syndrome. JPEN J Parenter Enteral Nutr 1991; 15(3): 347-9. [http://dx.doi.org/10.1177/0148607191015003347] [PMID: 1865554] |
| [100] | Volk DM, Cutliff SA. Selenium deficiency and cardiomyopathy in a patient with cystic fibrosis. J Ky Med Assoc 1986; 84(5): 222-4. [PMID: 3722990] |