- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

The Open Nutrition Journal
(Discontinued)
ISSN: 1874-2882 ― Volume 15, 2021
Nutritional Management of Cow's Milk Allergy in Infants: A Comparison of DRACMA, ESPGHAN, and AAP Guidelines
Emely L. Barrera1, Carlett Ramirez-Farias2, Barbara J. Marriage2, *
Abstract
Cow’s Milk Allergy (CMA) is one of the most common food allergies presented during infancy and childhood. The diagnosis and management of CMA is a complex task. First and foremost, CMA is manifested by a variety of symptoms classified by their type of mediation (either IgE and/or non-IgE responses), organ systems involved, and the onset of the reaction. Second, although several guidelines for the management of CMA have been published worldwide, they differ in their recommendations. To our knowledge, no global consensus exists for the management of the different symptoms associated with CMA. This review provides a table to compare three widely accepted published guidelines to enable the reader to easily navigate and compare the nutritional recommendations to be followed depending on the symptomatology. This review is intended to represent a practical tool to assess the nutritional recommendations for the management of CMA.
Article Information
Identifiers and Pagination:
Year: 2021Volume: 15
First Page: 1
Last Page: 9
Publisher Id: TONUTRJ-15-1
DOI: 10.2174/1874288202115010001
Article History:
Received Date: 18/8/2020Revision Received Date: 18/1/2021
Acceptance Date: 20/1/2021
Electronic publication date: 16/04/2021
Collection year: 2021
open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
* Address correspondence to this author at Scientific and Medical Affairs, Abbott Nutrition, Columbus, Ohio; Tel: +614-624-4416;
E-mail: barbara.marriage@abbott.com
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 18-8-2020 |
Original Manuscript | Nutritional Management of Cow's Milk Allergy in Infants: A Comparison of DRACMA, ESPGHAN, and AAP Guidelines | |
1. INTRODUCTION
The nutritional management of infants and children diagnosed with Cow’s Milk Allergy (CMA) is a complex task for healthcare practitioners. Contributing factors for the complexity are related to [1Boyce JA, Assa’ad A, Burks AW, et al. NIAID-Sponsored Expert Panel. Guidelines for the diagnosis and management of food allergy in the United States: Report of the NIAID-sponsored expert panel. J Allergy Clin Immunol 2010; 126(6)(Suppl.): S1-S58.
[http://dx.doi.org/10.1016/j.jaci.2010.10.008] [PMID: 21134576] ] the broad spectrum of CMA clinical presentations which vary by severity (mild to severe), and mediation type (immunoglobulin E [IgE], non-immunoglobulin E [non-IgE], or mixed) [1Boyce JA, Assa’ad A, Burks AW, et al. NIAID-Sponsored Expert Panel. Guidelines for the diagnosis and management of food allergy in the United States: Report of the NIAID-sponsored expert panel. J Allergy Clin Immunol 2010; 126(6)(Suppl.): S1-S58.
[http://dx.doi.org/10.1016/j.jaci.2010.10.008] [PMID: 21134576] , 2Muraro A, Werfel T, Hoffmann-Sommergruber K, et al. EAACI Food Allergy and Anaphylaxis Guidelines Group. EAACI food allergy and anaphylaxis guidelines: Diagnosis and management of food allergy. Allergy 2014; 69(8): 1008-25.
[http://dx.doi.org/10.1111/all.12429] [PMID: 24909706] ]; and [2Muraro A, Werfel T, Hoffmann-Sommergruber K, et al. EAACI Food Allergy and Anaphylaxis Guidelines Group. EAACI food allergy and anaphylaxis guidelines: Diagnosis and management of food allergy. Allergy 2014; 69(8): 1008-25.
[http://dx.doi.org/10.1111/all.12429] [PMID: 24909706] ] the differences in published nutritional management guidelines by medical organizations. Hence, there is a need for a resource that, in a practical manner, presents, compares, contrasts, and interprets the nutritional recommendations for infants and children diagnosed with CMA based on international consensus guidelines. In this article, the nutritional management of CMA is reviewed, and a practical comparison table is presented. Such a resource aims to assist healthcare practitioners in choosing the most suitable evidence-based nutritional management for infants and children diagnosed with CMA.
1.1. Cow’s Milk Allergy
CMA is the most common food allergy in children under 5 years of age [3American College of Asthma and Immunology: Milk allergy affects half of US food-allergic kids under age 1: Most children with a milk allergy don’t carry epinephrine. ScienceDaily 2020. Available at: https://www.science daily.com/releases/2018/11/181116083208.htm]. It is defined as an abnormal and undesired immune response triggered in a sensitized individual after exposure to Cow’s Milk Proteins (CMPs), typically casein and whey proteins such as β-lactoglobulin [4Jo J, Garssen J, Knippels L, Sandalova E. Role of cellular immunity in cow’s milk allergy: Pathogenesis, tolerance induction, and beyond. Mediators Inflamm 2014; 2014249784
[http://dx.doi.org/10.1155/2014/249784] [PMID: 25002754] ]. Cow’s milk, however, contains more than 20 protein allergens prone to cause reactions, and although casein fractions (α-S1-casein, α-S2-casein, β-casein) and β-lactoglobulin are the main allergens in cow’s milk, reactions to Bovine Serum Albumin (BSA) and α-lactalbumin have also been reported [5Elagamy EI. Milk Protein Allergy Reference Module in Food Sciences 2015; 1-5.]. In the United States alone, it is estimated that 2% of the population under 5 years of age [3American College of Asthma and Immunology: Milk allergy affects half of US food-allergic kids under age 1: Most children with a milk allergy don’t carry epinephrine. ScienceDaily 2020. Available at: https://www.science daily.com/releases/2018/11/181116083208.htm](464,000 children) [6Duffin E. Number of children aged between 0-5 years in the US 2000-2020 Statista 2019. Available at: https://www.statista.com/statistics/690946/ children-zero-to-five-years-of-age-america-us/] have CMA. Based on different cohort studies, the prevalence of CMA ranges between 1.9% and 7.5% for all infants and children [7Høst A, Halken S, Jacobsen HP, Christensen AE, Herskind AM, Plesner K. Clinical course of cow’s milk protein allergy/intolerance and atopic diseases in childhood. Pediatr Allergy Immunol 2002; 13(s15): 23-8.
[http://dx.doi.org/10.1034/j.1399-3038.13.s.15.7.x] [PMID: 12688620] -12Flom JD, Sicherer SH. Epidemiology of Cow’s Milk Allergy. Nutrients 2019; 11(5): 1051.
[http://dx.doi.org/10.3390/nu11051051] [PMID: 31083388] ].
According to the European Society for Paediatric Gastroenterology Hepatology and Nutrition (ESPGHAN), it is estimated that about 50% of children with CMA will develop tolerance to CMPs by the age of 12 months, more than 75% by the age of 3 years, and more than 90% by the age of 6 years [13Koletzko S, Niggemann B, Arató A, et al. Diagnostic approach and management of cow's-milk protein allergy in infants and children: ESPGHAN GI Committee practical guidelines. J Pediatric Gastroenterol Nutrition 2012; 55(2): 221-9.]. Two years prior to the publication of the ESPGHAN guidelines for the management of CMA, the World Allergy Organization’s (WAO) Diagnosis and Rationale for Action against Cow’s Milk Allergy (DRACMA) guidelines provided information on CMP tolerance development in children from two populations: (1) general public with no medical treatment before the study; and (2) referral patients with medical treatment before the study. Study findings showed that 56% of children develop CMP tolerance by the age of 1 year [14Fiocchi A, Brozek J, Schünemann H, et al. World Allergy Organization (WAO) diagnosis and rationale for action against cow’s milk allergy (DRACMA) guidelines. World Allergy Organ J 2010; 3(4): 57-161.
[http://dx.doi.org/10.1097/WOX.0b013e3181defeb9] [PMID: 23268426] ]. Furthermore, 77%, 87%, 92%, 92%, and 97% of children may develop CMP tolerance by the age of 2, 3, 5, 10, and 15 years, respectively [14Fiocchi A, Brozek J, Schünemann H, et al. World Allergy Organization (WAO) diagnosis and rationale for action against cow’s milk allergy (DRACMA) guidelines. World Allergy Organ J 2010; 3(4): 57-161.
[http://dx.doi.org/10.1097/WOX.0b013e3181defeb9] [PMID: 23268426] ].
CMA is mediated by 3 mechanisms: immunoglobulin E-mediated (IgE-mediated), non-IgE-mediated, and a mix of these two, each of which manifests a different set of symptoms. The symptoms are classified by severity, onset of the reaction (immediate or delayed), and the organ systems involved (respiratory, integumentary, gastrointestinal) [GI]. IgE-mediated reactions occur immediately or up to 2 hours after allergen exposure; whereas, non-IgE-mediated reactions may have a delayed response up to several days or weeks after allergen consumption [15Allen KJ, Davidson GP, Day AS, et al. Management of cow’s milk protein allergy in infants and young children: An expert panel perspective. J Paediatr Child Health 2009; 45(9): 481-6.
[http://dx.doi.org/10.1111/j.1440-1754.2009.01546.x] [PMID: 19702611] ]. For this reason, non-IgE reactions in CMA can be misdiagnosed and confused with lactose intolerance, gastroesophageal reflux disease (GERD), and other GI disorders. These reactions may require further elimination of the allergen from the diet coupled with oral food challenges to determine if the infant/child has an allergy to CMP [13Koletzko S, Niggemann B, Arató A, et al. Diagnostic approach and management of cow's-milk protein allergy in infants and children: ESPGHAN GI Committee practical guidelines. J Pediatric Gastroenterol Nutrition 2012; 55(2): 221-9.]. There are also clinical conditions where infants/children have a mix of IgE-mediated and non-IgE-mediated responses, such as atopic dermatitis and eosinophilic disorders [1Boyce JA, Assa’ad A, Burks AW, et al. NIAID-Sponsored Expert Panel. Guidelines for the diagnosis and management of food allergy in the United States: Report of the NIAID-sponsored expert panel. J Allergy Clin Immunol 2010; 126(6)(Suppl.): S1-S58.
[http://dx.doi.org/10.1016/j.jaci.2010.10.008] [PMID: 21134576] ].
Recognizing and implementing the best practices in CMA nutritional management by healthcare practitioners and caregivers is important to achieve optimal nutritional status, growth, and development. Symptom resolution is vital to achieve these outcomes. Practical tools, updated reviews, and accurate information describing the most optimal feeding methods based on the symptoms manifested by the infant/child are of utmost importance. Table 1 provides a summary of the most common CMA symptoms and clinical presentations.
1.2. Nutritional Alternatives for the Management of CMA
1.2.1. Breast Feeding
In general, it is widely recognized that to avoid the occurrence of CMA symptoms - “the strict avoidance of cow’s milk protein is the safest strategy” [13Koletzko S, Niggemann B, Arató A, et al. Diagnostic approach and management of cow's-milk protein allergy in infants and children: ESPGHAN GI Committee practical guidelines. J Pediatric Gastroenterol Nutrition 2012; 55(2): 221-9.-17Moissidis I, Chaidaroon D, Vichyanond P, Bahna SL. Milk-induced pulmonary disease in infants (Heiner syndrome). Pediatr Allergy Immunol 2005; 16(6): 545-52.
[http://dx.doi.org/10.1111/j.1399-3038.2005.00291.x] [PMID: 16176405] ]. Human milk is the gold standard for infant nutrition during the first 6 months of age and continued at least until 12 months of age. It contains nutrients required by the infant for energy, growth and metabolism, and non-nutritional components that together promote infant health, growth and development. Due to the unique benefits of human milk, in breastfed (BF) infants that present with CMA symptoms, the mother is advised to try a CMP-free diet and evaluate the infant to see if there is an improvement. It should be noted that it may take up to 72 hours for breast milk antigens to clear [18Lifschitz C, Szajewska H. Cow’s milk allergy: Evidence-based diagnosis and management for the practitioner. Eur J Pediatr 2015; 174(2): 141-50.
[http://dx.doi.org/10.1007/s00431-014-2422-3] [PMID: 25257836] ]. In addition, milk protein avoidance is recommended for two weeks and up to four weeks in cases of allergic colitis or atopic eczema, according to the DRACMA guidelines [14Fiocchi A, Brozek J, Schünemann H, et al. World Allergy Organization (WAO) diagnosis and rationale for action against cow’s milk allergy (DRACMA) guidelines. World Allergy Organ J 2010; 3(4): 57-161.
[http://dx.doi.org/10.1097/WOX.0b013e3181defeb9] [PMID: 23268426] ]. Eliminating the trigger foods from the mother’s diet usually results in gradual resolution of symptoms and enables the continuation of BF [19American Academy of Pediatrics Committee on Nutrition. Kleinman, R E & Greer, F R Pediatric nutrition : Policy of the American Academy of Pediatrics 7th ed. 2014; 981-1003.]. Occasionally, symptoms of food allergy do not resolve after extensive and strict elimination of foods in the mother’s diet [13Koletzko S, Niggemann B, Arató A, et al. Diagnostic approach and management of cow's-milk protein allergy in infants and children: ESPGHAN GI Committee practical guidelines. J Pediatric Gastroenterol Nutrition 2012; 55(2): 221-9.-15Allen KJ, Davidson GP, Day AS, et al. Management of cow’s milk protein allergy in infants and young children: An expert panel perspective. J Paediatr Child Health 2009; 45(9): 481-6.
[http://dx.doi.org/10.1111/j.1440-1754.2009.01546.x] [PMID: 19702611] , 18Lifschitz C, Szajewska H. Cow’s milk allergy: Evidence-based diagnosis and management for the practitioner. Eur J Pediatr 2015; 174(2): 141-50.
[http://dx.doi.org/10.1007/s00431-014-2422-3] [PMID: 25257836] ]. Several explanations have been hypothesized: the extent of or adherence to maternal food elimination diet is not enough, symptoms are not related to food allergy, and/or the infant could be reacting to endogenous human milk proteins. Although this is rare, the clinical experience is that these infant’s symptoms resolve only after discontinuation of BF and initiation of hypoallergenic formula [18Lifschitz C, Szajewska H. Cow’s milk allergy: Evidence-based diagnosis and management for the practitioner. Eur J Pediatr 2015; 174(2): 141-50.
[http://dx.doi.org/10.1007/s00431-014-2422-3] [PMID: 25257836] ].
1.2.2. Formula Feeding
In the absence of human milk, infant formulas are the most appropriate substitutes. A wide selection of infant formulas is available in the market with different macronutrient (carbohydrates, lipids, proteins) profiles. For the purpose of this article, we will discuss the differences based on the type of protein contained in the formulas.
-
Intact cow’s milk protein-based formula: These formulas are used for routine feeding of healthy term infants, to supplement breastmilk, or when BF is not available. Since these formulas contain intact (whole) protein, they should not be used to manage infants with CMA [18Lifschitz C, Szajewska H. Cow’s milk allergy: Evidence-based diagnosis and management for the practitioner. Eur J Pediatr 2015; 174(2): 141-50.
[http://dx.doi.org/10.1007/s00431-014-2422-3] [PMID: 25257836] ]. -
Partially hydrolyzed protein-based formula (pHF): These are formulas where the protein has been partially hydrolyzed (broken down). They are often used as an alternative to intact cow’s milk protein-based formula for mild intolerance symptoms such as fussiness and gas. Because the protein in these formulas is not extensively hydrolyzed, they could still cause an allergic reaction and therefore are contraindicated in the nutritional management of infants with diagnosed CMA [13Koletzko S, Niggemann B, Arató A, et al. Diagnostic approach and management of cow's-milk protein allergy in infants and children: ESPGHAN GI Committee practical guidelines. J Pediatric Gastroenterol Nutrition 2012; 55(2): 221-9., 18Lifschitz C, Szajewska H. Cow’s milk allergy: Evidence-based diagnosis and management for the practitioner. Eur J Pediatr 2015; 174(2): 141-50.
[http://dx.doi.org/10.1007/s00431-014-2422-3] [PMID: 25257836] ]. -
Soy protein-based formula (SF): SF is a lactose-free option and is recommended in infants with galactosemia and other lactase deficiency disorders, as well as in families with dietary restrictions (vegetarian/vegan diet) [18Lifschitz C, Szajewska H. Cow’s milk allergy: Evidence-based diagnosis and management for the practitioner. Eur J Pediatr 2015; 174(2): 141-50.
[http://dx.doi.org/10.1007/s00431-014-2422-3] [PMID: 25257836] ]. It is important to mention that lactase deficiency and lactose intolerance are not food allergies, and as such, are beyond the scope of this article.- Some guidelines give specific indications on when to use and not to use SF in infants with CMA.
-
Extensively hydrolyzed protein-based formula (EHF): An extensively hydrolyzed protein means that the protein has been broken down into small peptides and amino acids to virtually eliminate allergic reactions in most infants allergic to CMP. The American Academy of Pediatrics (AAP) defines that for a formula to be considered “hypoallergenic”, it must demonstrate in clinical studies with 95% confidence that the formula does not provoke allergic reactions in 90% of infants or children with confirmed CMA under prospective randomized, double-blind, placebo-controlled trials [24Baker S, Cochran W, Greer F, et al. Hypoallergenic infant formulas. Pediatrics 2000; 106(2 Pt 1): 346-9.
[PMID: 10920165] ]. It is important to highlight that although most infants with CMA tolerate hypoallergenic formulas with extensively hydrolyzed protein, some infants may require an amino acid-based formula (AAF) [14Fiocchi A, Brozek J, Schünemann H, et al. World Allergy Organization (WAO) diagnosis and rationale for action against cow’s milk allergy (DRACMA) guidelines. World Allergy Organ J 2010; 3(4): 57-161.
[http://dx.doi.org/10.1097/WOX.0b013e3181defeb9] [PMID: 23268426] , 15Allen KJ, Davidson GP, Day AS, et al. Management of cow’s milk protein allergy in infants and young children: An expert panel perspective. J Paediatr Child Health 2009; 45(9): 481-6.
[http://dx.doi.org/10.1111/j.1440-1754.2009.01546.x] [PMID: 19702611] ]. -
Amino acid-based formula (AAF): These formulas contain single (free) amino acids. They are designed for infants with extreme protein hypersensitivity and in cases where symptoms persist with an EHF feeding. The AAP advises that AAF go through clinical testing to confirm safety, tolerance, appropriate growth and development, and hypoallergenicity in clinical trials [14Fiocchi A, Brozek J, Schünemann H, et al. World Allergy Organization (WAO) diagnosis and rationale for action against cow’s milk allergy (DRACMA) guidelines. World Allergy Organ J 2010; 3(4): 57-161.
[http://dx.doi.org/10.1097/WOX.0b013e3181defeb9] [PMID: 23268426] ]. Fig. (1) illustrates protein structure in infant formulas and risk for allergic reaction in infants/children with CMA. - Rice Hydrolyzed Formula (RHF): RHFs are a class of plant-based infant feeding alternatives for special medical purposes composed of hydrolysates of rice protein. RHFs have been in the European market since the year 2000 but are not available in the United States.
2. METHODOLOGY
In line with the objective of this article, a comprehensive electronic search using scientific databases (i.e., PubMed, Google Scholar) was conducted. A specific inclusion criterion was applied to obtain adequate information and consisted of the following keywords: cow's milk allergy, guidelines, treatment, management, official, and infant formula. The search results were originally focused on all CMA guidelines. Some examples of country’s guidelines and/or consensus statements that were reviewed were from Belgium, Argentina, Turkey, Brasil, Middle East and Latin America. Most countries have used DRACMA, ESPGHAN, or a combination of both guidelines as a reference, with their own local adaptations or consensus. The three main guidelines chosen for this review have served as a template for multiple countries to create their own. For this reason, the focus was to review the three main guidelines (DRACMA, ESPGHAN, and AAP) for the management, diagnosis, and treatment of cow's milk allergy (CMA) that extensively discussed infant formula choices. The description of evidence is presented in a tabular format and further contrasted and discussed.
3. CMA CONSENSUS GUIDELINES
Diverse consensus guidelines have been published to identify and describe the feeding recommendations that are appropriate for the management of CMA. From 2000 to 2019, several global guidelines have been published to aid healthcare professionals in selecting nutritional strategies for infants and children diagnosed with CMA. Due to the international nature of these guidelines, some dietary recommendations for specific CMA symptoms vary between regions. This article reviews three of the most utilized CMA guidelines: (1) DRACMA (Diagnosis and Rationale for Action against Cow’s Milk Allergy) World Allergy Organization (WAO) Guidelines (2010) [14Fiocchi A, Brozek J, Schünemann H, et al. World Allergy Organization (WAO) diagnosis and rationale for action against cow’s milk allergy (DRACMA) guidelines. World Allergy Organ J 2010; 3(4): 57-161.
[http://dx.doi.org/10.1097/WOX.0b013e3181defeb9] [PMID: 23268426] ]; (2) ESPGHAN (European Society for Paediatric Gastroenterology, Hepatology and Nutrition) CMA Practical Guidelines (2012) [13Koletzko S, Niggemann B, Arató A, et al. Diagnostic approach and management of cow's-milk protein allergy in infants and children: ESPGHAN GI Committee practical guidelines. J Pediatric Gastroenterol Nutrition 2012; 55(2): 221-9.]; and (3) Policy statement on Hypoallergenic Infant Formulas of the American Academy of Pediatrics (AAP) (2000) [24Baker S, Cochran W, Greer F, et al. Hypoallergenic infant formulas. Pediatrics 2000; 106(2 Pt 1): 346-9.
[PMID: 10920165] ]. These three guidelines are the foundation for other CMA management guidelines established by various countries.
This article focuses on nutritional management practices for infants/children diagnosed with CMA. As the article summarizes only the most relevant nutritional management practices, it does not contain the supporting clinical evidence thoroughly discussed and analyzed in the three published guidelines. It is important to highlight that guidelines are not intended to impose a standard of care or substitute for individual clinical assessment but are available to provide a basis for clinical decisions. Strong recommendations based on high-quality evidence will apply to most of the patient population. No single recommendation can consider all unique clinical circumstances.
For healthcare professionals, it would be beneficial to have a practical and accessible resource, such as a comparative decision-making table that briefly outlines nutritional feeding recommendations to manage CMA depending on the symptoms and clinical presentations. The comparative table offered in this article allows for quick visualization of the similarities and differences between the three widely used guidelines. In addition, this article provides an updated review of the latest publication, “Cow’s milk allergy: towards an update of DRACMA guidelines” [25Fiocchi A, Dahda L, Dupont C, Campoy C, Fierro V, Nieto A. Cow’s milk allergy: Towards an update of DRACMA guidelines. World Allergy Organ J 2016; 9(1): 35.
[http://dx.doi.org/10.1186/s40413-016-0125-0] [PMID: 27895813] ].
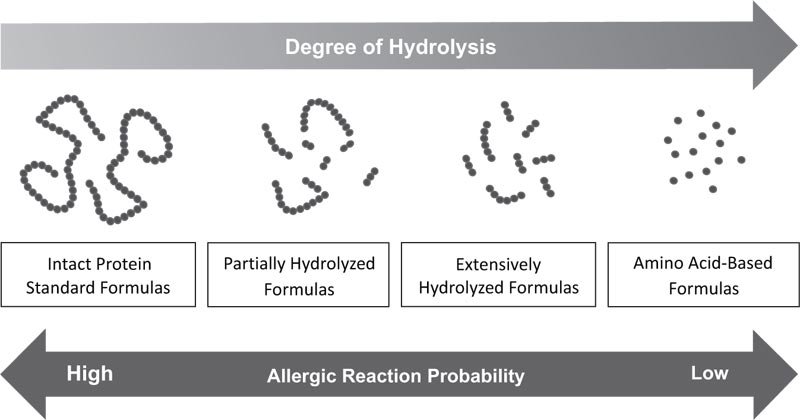 |
Fig. (1) Protein structure in infant formulas and risk for allergic reaction in infants/children with CMA. |
3.1. DRACMA Guidelines
In 2008, WAO Special Committee on Food Allergy recognized the need for an evidence-based approach to manage CMA. This committee conducted a systematic review of CMA literature, and used the GRADE (Grading of Recommendations, Assessment, Development and Evaluation) methodology for evaluating the quality of evidence. In this system, the quality of evidence is assessed based on explicit methodological criteria and classified as either “high,” “moderate,” “low,” or “very low.” Formulation of the recommendations within the guidelines included consideration of the quality of evidence, benefits, harms, burden, cost and values/preferences. After the GRADE approach, the guideline panelists classified CMA recommendations as either “strong” or “conditional” (which is considered weak). In 2010, the DRACMA Guidelines were first published, [14Fiocchi A, Brozek J, Schünemann H, et al. World Allergy Organization (WAO) diagnosis and rationale for action against cow’s milk allergy (DRACMA) guidelines. World Allergy Organ J 2010; 3(4): 57-161.
[http://dx.doi.org/10.1097/WOX.0b013e3181defeb9] [PMID: 23268426] ] with an update article in 2016 [25Fiocchi A, Dahda L, Dupont C, Campoy C, Fierro V, Nieto A. Cow’s milk allergy: Towards an update of DRACMA guidelines. World Allergy Organ J 2016; 9(1): 35.
[http://dx.doi.org/10.1186/s40413-016-0125-0] [PMID: 27895813] ]. The nutritional recommendations discussed in both documents are presented here.
The 2016 DRACMA publication continues to recognize the importance of breast milk for optimal nutrition and development of infants. When an infant is diagnosed with CMA, the pediatrician must recommend an “avoidance regimen”, which will substitute the infant’s diet with either an infant formula, or preferably, breastmilk with a mother’s diet free of CMP products [25Fiocchi A, Dahda L, Dupont C, Campoy C, Fierro V, Nieto A. Cow’s milk allergy: Towards an update of DRACMA guidelines. World Allergy Organ J 2016; 9(1): 35.
[http://dx.doi.org/10.1186/s40413-016-0125-0] [PMID: 27895813] ].
If the formula is warranted, the formula choice will depend on the infant’s symptoms and clinical presentation, as well as the availability of infant formulas in the market and financial resources of the family. DRACMA recommends EHF as option 1 for the treatment of non-severe and non-life-threatening CMA symptoms, such as immediate GI allergy, asthma, rhinitis, acute urticaria, angioedema, atopic dermatitis, CMP-induced enteropathy, GERD, constipation, severe irritability, colic, and CMP-induced gastroenteritis and proctocolitis. These guidelines do not specify the length of time the infant/child should consume the EHF before conducting an oral challenge to milk. However, they express that in earlier cohort studies using double-blind placebo-controlled food challenges (DBPCFC), 23% of infants suffering from CMA acquire tolerance after 13 months, and 75% after 43 months [14Fiocchi A, Brozek J, Schünemann H, et al. World Allergy Organization (WAO) diagnosis and rationale for action against cow’s milk allergy (DRACMA) guidelines. World Allergy Organ J 2010; 3(4): 57-161.
[http://dx.doi.org/10.1097/WOX.0b013e3181defeb9] [PMID: 23268426] ]. This could be an indicator of the advised consumption length of a hypoallergenic formula depending on the symptoms. The guidelines remark that SF should only be used in infants older than 6 months. In infants presenting with non-severe and non-life-threatening symptoms, SF is option 2 of treatment. If the infant is at risk of sensitization to soy proteins, AAF is recommended.
For life-threatening CMA reactions such as anaphylaxis (with a negative Skin Prick Test [SPT] to a specific formula), FPIES (food protein-induced enterocolitis syndrome), Heiner syndrome, and allergic eosinophilic esophagitis, AAF is option 1 of treatment followed by EHF as option 2. Upon availability, RHF can be a substitute for EHF when the symptoms are non-severe and non-life threatening. Several studies, summarized by Bocquet et al. (2019), [26Bocquet A, Dupont C, Chouraqui JP, et al. Efficacy and safety of hydrolyzed rice-protein formulas for the treatment of cow’s milk protein allergy. Arch Pediatr 2019; 26(4): 238-46.
[http://dx.doi.org/10.1016/j.arcped.2019.03.001] [PMID: 30979632] ] have reported the safety and effectiveness of RHF in managing CMA and other gastrointestinal disorders (i.e., secondary lactase deficiency and chronic or acute diarrhea). The 2010 guidelines published by DRACMA recommended further research to be performed with RHF [14Fiocchi A, Brozek J, Schünemann H, et al. World Allergy Organization (WAO) diagnosis and rationale for action against cow’s milk allergy (DRACMA) guidelines. World Allergy Organ J 2010; 3(4): 57-161.
[http://dx.doi.org/10.1097/WOX.0b013e3181defeb9] [PMID: 23268426] ]. In 2016, DRACMA published an update including new studies, and it was mentioned that upon availability, RHF could be a substitute for EHF when the symptoms are non-severe and non-life-threatening. If there is an anaphylactic reaction, RHF can substitute EHF as option 2 of treatment [25Fiocchi A, Dahda L, Dupont C, Campoy C, Fierro V, Nieto A. Cow’s milk allergy: Towards an update of DRACMA guidelines. World Allergy Organ J 2016; 9(1): 35.
[http://dx.doi.org/10.1186/s40413-016-0125-0] [PMID: 27895813] ]. SF is also option 2 to treat Heiner syndrome (after an AAF as option 1).
3.2. ESPGHAN Guidelines
In April 2012, ESPGHAN published the Diagnostic Approach and Management of Cow’s Milk Protein Allergy in Infants and Children: ESPGHAN GI Committee Practical Guidelines [13Koletzko S, Niggemann B, Arató A, et al. Diagnostic approach and management of cow's-milk protein allergy in infants and children: ESPGHAN GI Committee practical guidelines. J Pediatric Gastroenterol Nutrition 2012; 55(2): 221-9.]. The authors, reviewers, and researchers included a wide range of experts from various institutions. Like DRACMA, these guidelines present a set of recommendations for the diagnosis and management of suspected CMA. Based on the evidence, the ESPGHAN guidelines also provide an algorithm to diagnose CMA. The nutritional recommendations after a confirmed CMA diagnosis are presented below.
The ESPGHAN guidelines advise that BF should be strongly encouraged for infants with CMA. In BF infants, the mother should start a strict CMP-free diet coupled with calcium supplements (1000 mg/day spread across the day), complemented with additional nutrition counseling.
ESPGHAN recognizes that most infants/children will tolerate EHF with whey or casein as the protein source. The formula recommendations vary depending on the age of the infant/child as well as the severity of the reaction. In infants <12 months of age with CMA and non-life-threatening symptoms, EHF is option 1 for nutritional management of infants of at least 6 months of age or until 9-12 months of age. Nevertheless, if the symptoms are severe, then EHF is recommended for 12-18 months of age before conducting an oral challenge in the child. ESPGHAN recommends AAF as option 1 for the nutritional management of anaphylaxis, EoE, and severe enteropathy.
Like DRACMA, ESPGHAN does not recommend the use of SF in the first 6 months of age due to the high prevalence of cross-reactivity in young infants. However, after 6 months of age and in non-life-threatening scenarios, SF can be considered as option 2 in the following scenarios; if the infant manifests intolerance to EHF and AAF, if the latter two formulas are unaffordable, and in vegan families.
ESPGHAN recommends the use of RHF, but due to the limited availability in markets and limited data, the support for RHF use is limited to infants/children that are either refusing or not tolerating EHF, or in vegan families.
3.3. AAP and National Institute of Allergy and Infectious Diseases (NIAID) Guidelines
The AAP Committee on Nutrition published a policy statement in 2000, “Hypoallergenic Infant Formulas”, which provides recommendations on the use of different infant formulas for the management of CMA [24Baker S, Cochran W, Greer F, et al. Hypoallergenic infant formulas. Pediatrics 2000; 106(2 Pt 1): 346-9.
[PMID: 10920165] ]. In 2011, the NIAID published the “Guidelines for the Diagnosis and Management of Food Allergy in the United States” [1Boyce JA, Assa’ad A, Burks AW, et al. NIAID-Sponsored Expert Panel. Guidelines for the diagnosis and management of food allergy in the United States: Report of the NIAID-sponsored expert panel. J Allergy Clin Immunol 2010; 126(6)(Suppl.): S1-S58.
[http://dx.doi.org/10.1016/j.jaci.2010.10.008] [PMID: 21134576] ]. The NIAID guidelines are inclusive of all food allergies and are non-specific to CMA, hence, they only provide a general recommendation to use hypoallergenic formulas in infants with suspected CMA. NIAID gave general recommendations on the use of hypoallergenic formulas; therefore, it was decided to use the AAP policy statement on Hypoallergenic Infant Formulas in our comparative table as it more thoroughly discusses the nutritional recommendations. The AAP Committee on Nutrition stated that “The American Academy of Pediatrics is committed to BF as the ideal source of nutrition for infants” [24Baker S, Cochran W, Greer F, et al. Hypoallergenic infant formulas. Pediatrics 2000; 106(2 Pt 1): 346-9.
[PMID: 10920165] ]. This policy statement recognizes the importance of breastmilk as the ideal source of nutrition through the first 12 months of age or longer. When CMA is suspected, the maternal diet should be void of cow’s milk, egg, fish, peanuts, and tree nuts, and should be supplemented with calcium.
Infants who do not tolerate breastmilk despite the maternal avoidance of CMP may benefit from the use of a hypoallergenic formula such as EHF. If symptoms persist, then an AAF should be considered. The AAP policy statement remarks that when infants present with IgE-mediated reactions such as angioedema, urticaria, wheezing, rhinitis, vomiting, eczema, and even anaphylaxis, SF could be an option as an initial treatment or preferably after 6 months of age following the use of EHF or AAF [22Issues AAP. Recommendations for the Use of Soy Protein-Based Formulas in Infant Feeding - Special Medical Reports - American Family Physician. Am Fam Physician 1998; 57(11): 2876-6.
[PMID: 9636345] ]. Unlike IgE-mediated reactions, infants manifesting non-IgE-mediated symptoms such as enterocolitis, malabsorption syndrome, esophagitis, and proctocolitis should not use SF as the prevalence of concomitant reactions between soy and CMP is higher in infants (25% to 60%) with non-IgE-mediated symptoms [22Issues AAP. Recommendations for the Use of Soy Protein-Based Formulas in Infant Feeding - Special Medical Reports - American Family Physician. Am Fam Physician 1998; 57(11): 2876-6.
[PMID: 9636345] ]. RHF is not addressed by the AAP for a treatment option as it is not available in the United States. Milk from goats and other animals, or infant formulas containing large amounts of intact animal protein, are inappropriate alternatives for infants allergic to CMP.
4. DISCUSSION
Comparative tables featuring highlights of the DRACMA, ESPGHAN and AAP CMA guidelines are a time-efficient resource for healthcare practitioners in the nutritional management and selection of optimal feeding methods for infants and children with diagnosed CMA. Tables 2 and 3 illustrate a comparative review of the highlighted guidelines. Guidelines providing direct recommendations for specific clinical presentations/symptoms are marked in bold font in the tables. Guidelines providing generalized recommendations (i.e., extensively hydrolyzed formulas used as a first line of treatment) are the authors’ interpretation of the nutritional recommendations based on the allergic reaction severity and type of mediation per individual symptom and marked in normal font in Table 3.
It is important to mention that since ESPGHAN and AAP make general rather than symptom-specific recommendations to use EHF formula as a first line of treatment for CMA, a limitation of this review is that Table 3 includes author interpreted recommendations for specific symptoms (in normal font) to align with the clinical presentations discussed in the latest DRACMA publication [13Koletzko S, Niggemann B, Arató A, et al. Diagnostic approach and management of cow's-milk protein allergy in infants and children: ESPGHAN GI Committee practical guidelines. J Pediatric Gastroenterol Nutrition 2012; 55(2): 221-9., 14Fiocchi A, Brozek J, Schünemann H, et al. World Allergy Organization (WAO) diagnosis and rationale for action against cow’s milk allergy (DRACMA) guidelines. World Allergy Organ J 2010; 3(4): 57-161.
[http://dx.doi.org/10.1097/WOX.0b013e3181defeb9] [PMID: 23268426] , 24Baker S, Cochran W, Greer F, et al. Hypoallergenic infant formulas. Pediatrics 2000; 106(2 Pt 1): 346-9.
[PMID: 10920165] ].
The guidelines discussed in Tables 2 and 3 serve as a guide for the nutritional management of CMA. Although certain recommendations are similar between guidelines, some vary, which reflect cultural differences and regulations across different regions. It is important to note that all three guidelines recommend BF as the gold standard of infant nutrition and should be encouraged, when applicable, through the diagnosis and management stages of CMA. The guidelines emphasize that an effort must be made to maintain exclusive BF for at least the first 6 months of age, breastmilk as complementary feeding after weaning, and preferably during the first year of life and/or as long as possible.
Non-breastfed infants with CMA symptoms will require a hypoallergenic formula. Although there is no global consensus on the labeling of hypoallergenic formulas, the AAP advises that hypoallergenic formula “must demonstrate in clinical studies with 95% confidence that the formula does not provoke allergic reactions in 90% of infants or children with confirmed CMA under prospective randomized, double-blind, placebo-controlled trials” [24Baker S, Cochran W, Greer F, et al. Hypoallergenic infant formulas. Pediatrics 2000; 106(2 Pt 1): 346-9.
[PMID: 10920165] ]. Generally, the infant formulas that meet these criteria are EHF and AAF. AAP agrees on the use of SF in infants with documented IgE-associated allergy to cow’s milk who are not allergic to soy protein. On the contrary, DRACMA and ESPGHAN do not recommend SF in IgE-allergic reactions unless infants are older than 6 months of age.
Only the AAP guideline states that anaphylactic reactions to soy proteins are rare; therefore, the AAP mentions that SF can be fed, contingent to the patient not being at risk and not manifesting adverse reactions to soy. In EoE scenarios, there is not a specific recommendation for AAF from the AAP; instead, a general recommendation to use hypoallergenic formulas, with EHF as option 1 for the nutritional management, and then AAF as option 2 if there is a reaction to EHF. However, newer guidelines specific for certain disorders (i.e., FPIES, EoE) have been published to address more detailed nutritional management and treatment, but they will not be discussed in this article.
CONCLUSION
There are many similarities among DRACMA, ESPGHAN and AAP guidelines for the nutritional management of CMA-diagnosed infants. All guidelines strongly agree that breastmilk is the gold standard for infant nutrition, including CMA-diagnosed infants, in the first 6 to 12 months of life. When, BF is not possible, the three guidelines agree that EHF should be the first treatment option for mild-moderate symptoms. If the infant/child does not tolerate EHF, DRACMA, ESPGHAN, and AAP recommend the use of AAF. Exclusively, in severe CMA presentations (e.g., anaphylaxis, Heiner syndrome, FPIES, EoE, and severe enteropathy), AAF should be the first option of treatment (DRACMA and ESPGHAN). Both DRACMA and ESPGHAN recommend that in infants older than 6 months with IgE-mediated CMA, SF can be used if there is no cross-reactivity with CMP. Interestingly, AAP states that anaphylactic reactions to soy protein are extremely rare, and therefore, this statement does not eliminate the possibility of feeding SF to infants under 6 months of age with IgE-mediated allergy, except for those infants with demonstrated adverse reactions to soy proteins.
In terms of more novel nutritional options, such as plant-based infant formulas, DRACMA discusses the potential benefits of RHF as an option for the nutritional management of infants with mild and severe CMA allergic reactions. RHF in countries where it is available could represent a substitute for EHF in several clinical presentations. Finally, the three guidelines concur that pHF and other mammalian milks are not recommended for the nutritional management of CMA, and as such, should be avoided in infants where CMA has been diagnosed.
These guidelines have helped healthcare professionals in tailoring their nutritional management option, allowing them to take into consideration the individualized context of each patient, their values and preferences. Certainly, guidelines will keep evolving as new evidence emerges.
LIST OF ABBREVIATIONS
| AAF | = Amino Acid-based Formula |
| AAP | = American Academy of Pediatrics |
| BF | = Breast Feeding/Breastfed |
| Ca | = Calcium |
| CMA | = Cow’s Milk Allergy |
| CMP | = Cow’s Milk Protein |
| DRACMA | = Diagnosis and Rationale for Action Against Cow’s Milk Allergy |
| EHF | = Extensively Hydrolyzed Formula |
| EoE | = Eosinophilic Esophagitis |
| ESPGHAN | = European Society for Paediatric Gastroenterology, Hepatology and Nutrition |
| FF | = Formula-Fed |
| FPIES | = Food Protein-Induced Enterocolitis Syndrome |
| GERD | = Gastroesophageal Reflux Disease |
| GI | = Gastrointestinal |
| IgE | = Immunoglobulin E |
| Mo | = Months |
| NIAID | = National Institute of Allergy and Infectious Diseases |
| non-IgE | = non-immunoglobulinE |
| pHF | = Partially Hydrolyzed Formula |
| RHF | = Rice Hydrolyzed Formula |
| SF | = Soy Formula |
| SPT | = Skin Prick Test |
| WAO | = World Allergy Organization |
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors are employees of Abbott Nutrition. The content of this article has no conflict of interest.
ACKNOWLEDGEMENTS
Declared none.
REFERENCES
| [1] | Boyce JA, Assa’ad A, Burks AW, et al. NIAID-Sponsored Expert Panel. Guidelines for the diagnosis and management of food allergy in the United States: Report of the NIAID-sponsored expert panel. J Allergy Clin Immunol 2010; 126(6)(Suppl.): S1-S58. [http://dx.doi.org/10.1016/j.jaci.2010.10.008] [PMID: 21134576] |
| [2] | Muraro A, Werfel T, Hoffmann-Sommergruber K, et al. EAACI Food Allergy and Anaphylaxis Guidelines Group. EAACI food allergy and anaphylaxis guidelines: Diagnosis and management of food allergy. Allergy 2014; 69(8): 1008-25. [http://dx.doi.org/10.1111/all.12429] [PMID: 24909706] |
| [3] | American College of Asthma and Immunology: Milk allergy affects half of US food-allergic kids under age 1: Most children with a milk allergy don’t carry epinephrine. ScienceDaily 2020. Available at: https://www.science daily.com/releases/2018/11/181116083208.htm |
| [4] | Jo J, Garssen J, Knippels L, Sandalova E. Role of cellular immunity in cow’s milk allergy: Pathogenesis, tolerance induction, and beyond. Mediators Inflamm 2014; 2014249784 [http://dx.doi.org/10.1155/2014/249784] [PMID: 25002754] |
| [5] | Elagamy EI. Milk Protein Allergy Reference Module in Food Sciences 2015; 1-5. |
| [6] | Duffin E. Number of children aged between 0-5 years in the US 2000-2020 Statista 2019. Available at: https://www.statista.com/statistics/690946/ children-zero-to-five-years-of-age-america-us/ |
| [7] | Høst A, Halken S, Jacobsen HP, Christensen AE, Herskind AM, Plesner K. Clinical course of cow’s milk protein allergy/intolerance and atopic diseases in childhood. Pediatr Allergy Immunol 2002; 13(s15): 23-8. [http://dx.doi.org/10.1034/j.1399-3038.13.s.15.7.x] [PMID: 12688620] |
| [8] | Saarinen KM, Juntunen-Backman K, Järvenpää AL, et al. Supplementary feeding in maternity hospitals and the risk of cow’s milk allergy: A prospective study of 6209 infants. J Allergy Clin Immunol 1999; 104(2 Pt 1): 457-61. [http://dx.doi.org/10.1016/S0091-6749(99)70393-3] [PMID: 10452771] |
| [9] | Kvenshagen B, Halvorsen R, Jacobsen M. Adverse reactions to milk in infants. Acta Paediatr 2008; 97(2): 196-200. [http://dx.doi.org/10.1111/j.1651-2227.2007.00599.x] [PMID: 18254909] |
| [10] | Venter C, Pereira B, Grundy J, et al. Incidence of parentally reported and clinically diagnosed food hypersensitivity in the first year of life. J Allergy Clin Immunol 2006; 117(5): 1118-24. [http://dx.doi.org/10.1016/j.jaci.2005.12.1352] [PMID: 16675341] |
| [11] | Schrander JJ, van den Bogart JP, Forget PP, Schrander-Stumpel CT, Kuijten RH, Kester AD. Cow’s milk protein intolerance in infants under 1 year of age: A prospective epidemiological study. Eur J Pediatr 1993; 152(8): 640-4. [http://dx.doi.org/10.1007/BF01955238] [PMID: 8404966] |
| [12] | Flom JD, Sicherer SH. Epidemiology of Cow’s Milk Allergy. Nutrients 2019; 11(5): 1051. [http://dx.doi.org/10.3390/nu11051051] [PMID: 31083388] |
| [13] | Koletzko S, Niggemann B, Arató A, et al. Diagnostic approach and management of cow's-milk protein allergy in infants and children: ESPGHAN GI Committee practical guidelines. J Pediatric Gastroenterol Nutrition 2012; 55(2): 221-9. |
| [14] | Fiocchi A, Brozek J, Schünemann H, et al. World Allergy Organization (WAO) diagnosis and rationale for action against cow’s milk allergy (DRACMA) guidelines. World Allergy Organ J 2010; 3(4): 57-161. [http://dx.doi.org/10.1097/WOX.0b013e3181defeb9] [PMID: 23268426] |
| [15] | Allen KJ, Davidson GP, Day AS, et al. Management of cow’s milk protein allergy in infants and young children: An expert panel perspective. J Paediatr Child Health 2009; 45(9): 481-6. [http://dx.doi.org/10.1111/j.1440-1754.2009.01546.x] [PMID: 19702611] |
| [16] | Yukselen A, Celtik C. Food allergy in children with refractory gastroesophageal reflux disease. Pediatr Int (Roma) 2016; 58(4): 254-8. [http://dx.doi.org/10.1111/ped.12779] [PMID: 26257132] |
| [17] | Moissidis I, Chaidaroon D, Vichyanond P, Bahna SL. Milk-induced pulmonary disease in infants (Heiner syndrome). Pediatr Allergy Immunol 2005; 16(6): 545-52. [http://dx.doi.org/10.1111/j.1399-3038.2005.00291.x] [PMID: 16176405] |
| [18] | Lifschitz C, Szajewska H. Cow’s milk allergy: Evidence-based diagnosis and management for the practitioner. Eur J Pediatr 2015; 174(2): 141-50. [http://dx.doi.org/10.1007/s00431-014-2422-3] [PMID: 25257836] |
| [19] | American Academy of Pediatrics Committee on Nutrition. Kleinman, R E & Greer, F R Pediatric nutrition : Policy of the American Academy of Pediatrics 7th ed. 2014; 981-1003. |
| [20] | Rajani PS, Martin H, Groetch M, Järvinen KM. Presentation and management of food allergy in breastfed infants and risks of maternal elimination diets: Clinical management review. J Allergy Clin Immunol Pract 2020; 8(1): 52-67. [http://dx.doi.org/10.1016/j.jaip.2019.11.007] [PMID: 31751757] |
| [21] | Heyman MB. Lactose intolerance in infants, children, and adolescents. Pediatrics 2006; 118(3): 1279-86. [http://dx.doi.org/10.1542/peds.2006-1721] |
| [22] | Issues AAP. Recommendations for the Use of Soy Protein-Based Formulas in Infant Feeding - Special Medical Reports - American Family Physician. Am Fam Physician 1998; 57(11): 2876-6. [PMID: 9636345] |
| [23] | Bhatia J, Greer F. Use of soy protein-based formulas in infant feeding. Pediatrics 2008; 121(5): 1062-8. [http://dx.doi.org/10.1542/peds.2008-0564] [PMID: 18450914] |
| [24] | Baker S, Cochran W, Greer F, et al. Hypoallergenic infant formulas. Pediatrics 2000; 106(2 Pt 1): 346-9. [PMID: 10920165] |
| [25] | Fiocchi A, Dahda L, Dupont C, Campoy C, Fierro V, Nieto A. Cow’s milk allergy: Towards an update of DRACMA guidelines. World Allergy Organ J 2016; 9(1): 35. [http://dx.doi.org/10.1186/s40413-016-0125-0] [PMID: 27895813] |
| [26] | Bocquet A, Dupont C, Chouraqui JP, et al. Efficacy and safety of hydrolyzed rice-protein formulas for the treatment of cow’s milk protein allergy. Arch Pediatr 2019; 26(4): 238-46. [http://dx.doi.org/10.1016/j.arcped.2019.03.001] [PMID: 30979632] |
| [27] | Nowak-Węgrzyn A, Chehade M, Groetch ME, et al. International consensus guidelines for the diagnosis and management of food protein-induced enterocolitis syndrome: Executive summary-Workgroup report of the adverse reactions to foods committee, american academy of allergy, asthma & immunology. J Allergy Clin Immunol 2017; 139(4): 1111-1126.e4. [http://dx.doi.org/10.1016/j.jaci.2016.12.966] [PMID: 28167094] |