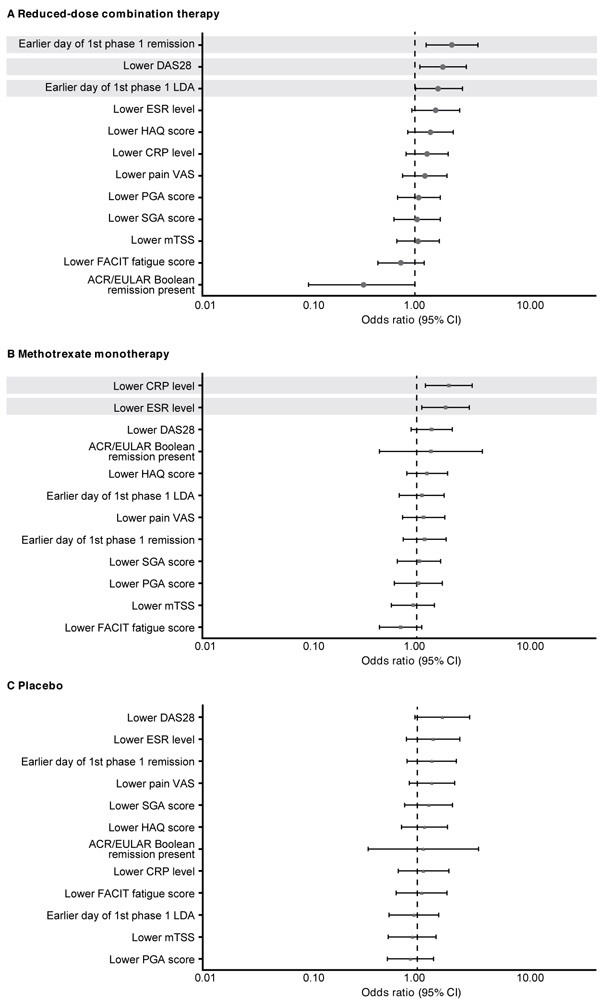
Fig. (3)
Treatment responses at week 52 as predictors of sustained remission in patients who received (A) reduced-dose combination therapy, (B) methotrexate monotherapy, and (C) placebo in the double-blind phase (double-blind mITT population). ACR/EULAR: American College of Rheumatology/European League Against Rheumatism; CI: Confidence Interval; CRP: C-Reactive Protein; DAS28: Disease Activity Score for 28-joint counts; ESR: Erythrocyte Sedimentation Rate; FACIT: Functional Assessment of Chronic Illness; HAQ: Health Assessment Questionnaire; LDA: Low Disease Activity; mITT: modified Intent-To-Treat; mTSS: modified Total Sharp Score; PGA: Physician Global Assessment; SGA: Subject Global Assessment; VAS: Visual Analog Scale.