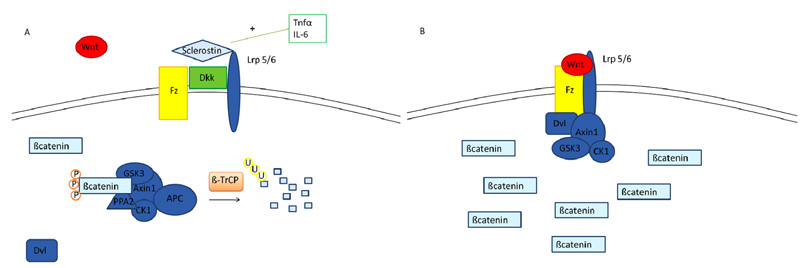
Fig. (2)
The figure shows Wnt/ß-catenin system. ß-catenin is normally degraded by a multiprotein complex, consisting of Axin1, Adenomatous Polyposis Coli (APC), GSK-3 and CK1, the protein phosphatase 2A (PP2A) and the E3-ubiquitin ligase β-TrCP; after ubiquitination, β-catenin is digested by the proteasome (A). When a member of the Wnt protein family becomes available, the transmembrane receptor Frizzled (Fz) and its coreceptors LRP 4, 5 or 6 (Lipoprotein Receptor-related Proteins) are activated and phosphorylated leading to the recruitment of the protein Disheveled (Dvl). Axin is seized and the degradation complex undergoes inhibitory rearrangements, leading to the accumulation of ß-catenin and its translocation to the nucleus where it acts as a co-transcription factor (B). Proinflammatory cyrokines are able to inhibit this pathway by enhanincg the production of inhibitory molecules like DKK and sclerostin, which prevent the interaction between Wnt and its receptors.