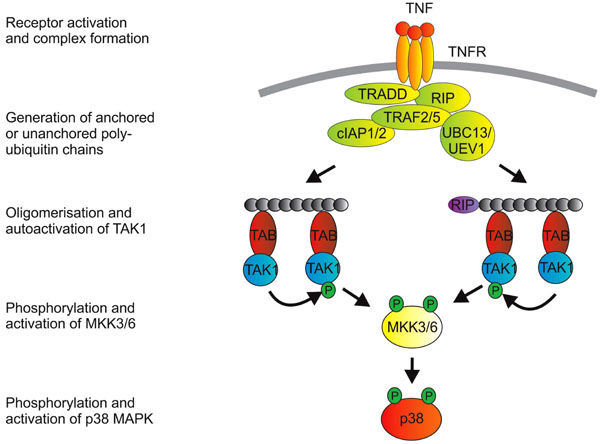
Fig. (1) Activation of p38 MAPK downstrem of TNFR1. Engagement of TNFR1 by trimeric TNF promotes a structural change in the
cytoplasmic domain of the receptor (which is itself trimeric). The cytoplasmic death domain of TNFR1 then mediates recruitment of the
adaptor protein TRADD (TNF receptor associated death domain protein). This provides a platform for recruitment of additional proteins that
include the kinase RIP1 (receptor interacting protein 1), TNF receptor associated factors (TRAFs) 2 and 5, cellular inhibitors of apoptosis
proteins (cIAPs) 1 and 2 and, it is thought, the E2 enzymes UBC13 and UEV1. From here, the picture of signaling events becomes a little
blurred, because: i) a number of entities in the complex possess ubiquitin E3 ligase activity (namely the TRAFs and the cIAPs); ii) some
ubiquitin modifications of proteins in the complex have not been consistently identified in different labs; and iii) downstream events have
often been studied in the context of signaling to NF-κB or JNK rather than p38 MAPK. K63-linked polyubiquitination of the TRAFs
themselves or of RIP have been implicated in downstream signaling. A more recent notion is that transmission of the signal depends on the
generation of unanchored polyubiquitin chains [166]. TAK1 is next recruited to the complex via interactions between polyubiquitin chains
and TAK1 binding proteins (TABs), which possess poly-ubiquitin binding domains [11]. TAK1 proteins that are brought together at
polyubiquitin chains then phosphorylate and activate one another. K63-linked ubiquitination of TAK1 itself may also be involved in the
activation of p38 MAPK [167].