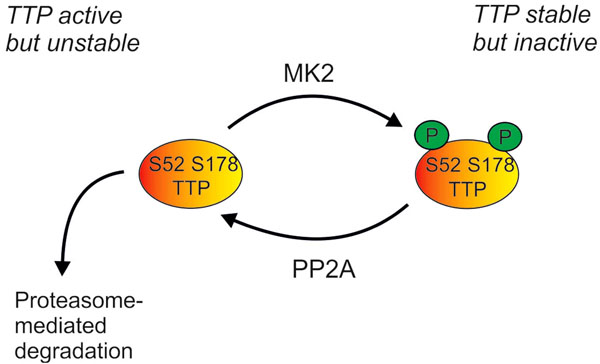
Fig. (3) Regulation of cellular levels of TTP protein by the p38 MAPK pathway. Phosphorylation of TTP by MK2 renders it inactive (as
described in Fig 2), but also protects it against proteasome-mediated degradation. TTP can be dephosphorylated by protein phosphatase 2A
(PP2A), and perhaps by other phosphatases. Cellular levels of TTP are therefore dependent on a dynamic equilibrium of protein synthesis,
proteasome-mediated degradation, MK2-mediated phosphorylation and PP2A-mediated dephosphorylation.