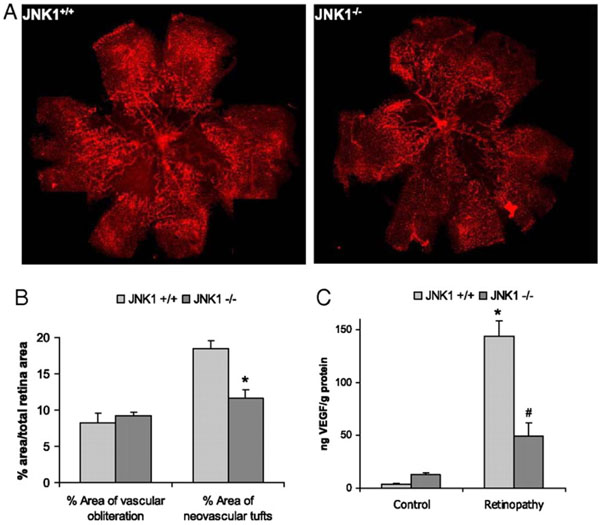
Fig. (2) JNK1 regulates VEGF expression and neo-vascularization in a murine model of retinopathy. To determinate whether JNK1
regulates VEGF production, we analyzed its role in oxygen-induced retinopathy (OIR), a well established model of retinopathy of
prematurity (ROP). In this model, when mouse pups are exposed to hyperoxia (75% oxygen) from postnatal day 7 to postnatal day 12 (from
P7 to P12), vessel regression and cessation of normal radial vessel growth occur. Upon return to ambient air (normoxia) from P12 to P17, the
non-perfused regions of the retina become hypoxic, resulting in expression of angiogenic factors, such as VEGF, and retinal
neovascularization. Thus, litters of WT and Jnk1-/- pups were placed under hyperoxia (75% oxygen) for 5 days on P7. After 5 days, on P12,
mice were returned to ambient air until P17, when they were sacrificed, their eyes were enucleated and retinas were isolated. (A) Whole
mounted retinas from mice exposed to hyperoxia followed by normoxia were stained with Alexa Fluor 594-conjugated B4 isolectin from
Griffonia simplicifolia, which labels endothelial cells, and viewed by fluorescent microscopy. (B) Areas of vascular obliteration and
neovascular tufts were quantified using at least 6 mice per genotype. Results are expressed as means ± s.e.m. * =p< 0.05 vs WT mice (C)
Retinal proteins were extracted at P17 and VEGF was quantified by ELISA. Results are averages of two experiments using at least 6 mice
per genotype. Results are expressed as means ± s.e.m. * =p< 0.05 vs control; #= p< 0.05 vs WT mice [19].