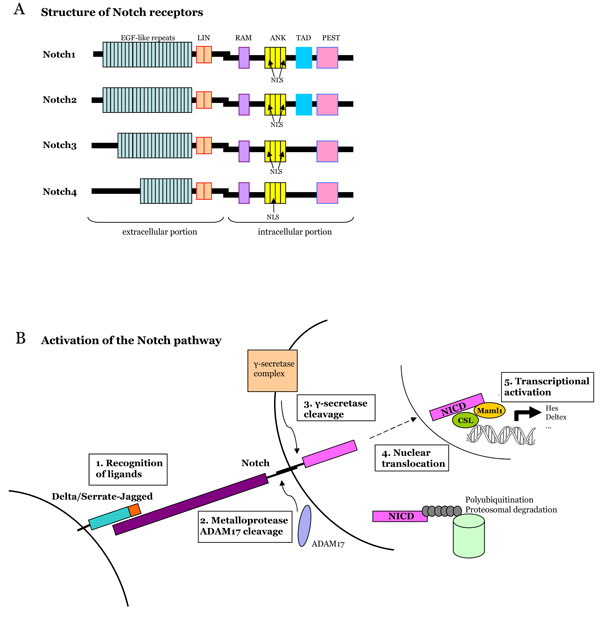
Fig. (1) A. Structure of Notch receptors in mammals. Notch is a heterodmeric transmembrane receptor. The extracellular domain
containsEGFlike repeats and LIN repeats. The intracellular domain contains a RAM domain (RBP-J associated molecule), nuclear
localization sequences (NLS), seven ankyrin repeats (ANK), a transactivation domain (TAD) and a PEST sequence (Proline, Glutamate,
Serine, Threonine-rich domain). The four Notch receptors differ in the number of EGF-like repeats and Cterminal sequences. B. Activation
of the Notch pathway. Notch binds its l igand, is first cleaved by a metalloprotease and second by the γ-secretase compex. These cleavages
allow the release of the Notch intra-cellular domain (NICD) and its transport into the nucleus, where it cooperates with transcription factors
to regulate gene activity.