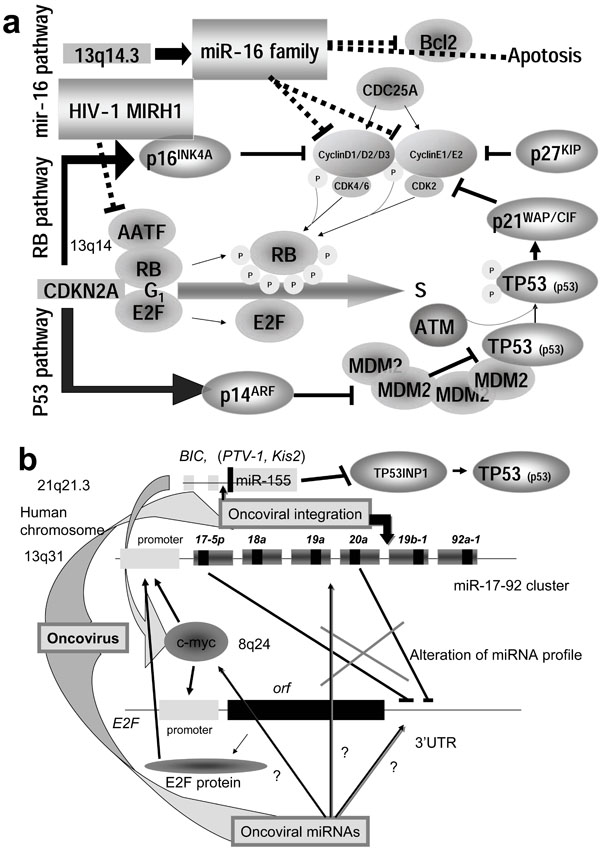
Fig. (3) Master regulator miRNA of oncogenic and tumor suppressor. (a) The tumor suppressor has two pathways, RB and p53 pathway. The miR-16 family regulates the cell cycle G1/S by modulating CDK4/6-Cyclin D1/D2/D3 and CDK2-Cyclin E1/E2 complexes. Two complexes are related to RB phosphorylation, and the phosphorylated RB is released from E2F. Thus the activated E2F drives infinitive transcription from G1 into S. Another player in carcinogenesis is CDKN2A, which encodes p16INK4A and p14ARF. The former is transcribed from exons 1α, 2 and 3, and the latter is from exon 1β, 2 and 3 with different reading frames of the CDKN2A gene. p16 inhibits the CDK4/6-Cyclin D complex and p14 mediates G1 arrest by destabilizing the MDM2 protein. The MDM2 oncogene induces many sarcomas by binding to p53 (TP53). Therefore, p14 maintains the level of p53. p53 phosphorylated by ATM inhibits CDK2/Cyclin E complex via p21WAP/CIP activation with p27KIP. The main mechanism of tumor suppression is intervention by CDK4/6-Cyclin D and CDK2-Cyclin E. miR-16 can induce apoptosis by suppression of Bcl2. Thus, the miR-16 family plays an important role for tumor suppression. Although viral oncoproteins, such as adenovirus E1A, SV40 T antigen, as well as human papillomavirus E7, bind RB, the HIV-1 viral MIRH-1 was recently shown to suppress AATF which inhibits E2F activation by association with RB. Therefore, oncoviral MIRH1 may be tumorigenic. (b) Cell cycle transcription by E2F is also modulated by a c-myc-regulated miRNA cluster miR-17-92 [138]. In the upper part of the figure showing the miR-17-92 cluster, the large box represents the pre-miRNA and the small box shows the mature miRNA. MYC (c-myc) promotes the transcription of both miR-17-92 and E2F by binding at the cacgtg site on the miR-17-92 gene and the E2F promoter. miR-17-5p and miR-20a downregulate E2F gene expression. miR-17-92 results in the inhibition of apoptosis via the p14ARF pathway. miR-155 oncogenic miRNA in oncoviral integration site BIC activates c-myc and inhibits p53 by inactivation of TP53INP1. In some cases, oncoviral integration upregulates miR-155 expression, but in other cases, oncoviral integration inhibits miR-17-92 expression. Thus, oncoviral integration is tumorigenic. However, no relation between integration site and miRNA genes has been reported. The primary cause of altered miRNA expression in cancer cells is still unaccounted for. While miR-125b-1 insertion into IGH locus has been found in acute lymphoblastic leukemia, if the oncovirus takes the resident miRNA as the MGE, the mobile miRNA may be able to alter the profiles of miRNA in infected cells. Subsequently, hidden oncoviral miRNAs may have an important role for tumorigenesis in the cells.