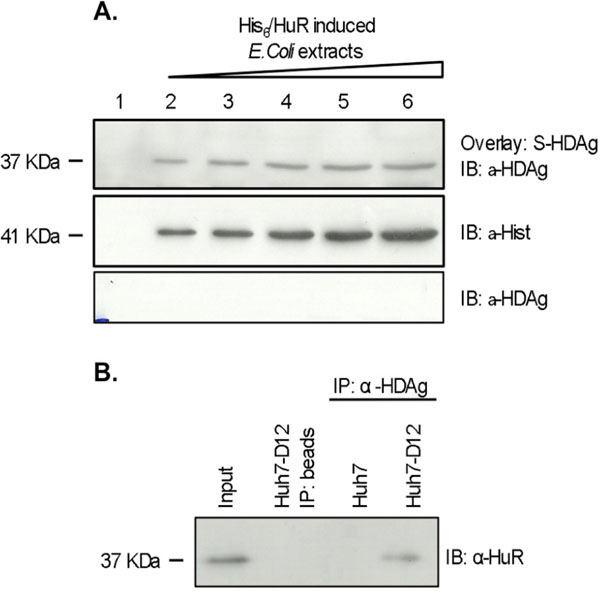
Fig. (2) In vitro and in vivo interaction of HuR and S-HDAg. (A) Overlay of bacterially expressed His6-HuR with recombinant S-HDAg. E. coli protein extracts were prepared after induction of recombinant protein expression. Increasing amounts of extracts containing His6-HuR (lanes 2 to 6), were separated by SDS/PAGE, blotted onto nitrocellulose membranes and overlayed with the same amount of purified recombinant S-HDAg. Detection of S-HDAg was performed with a specific rabbit polyclonal antibody. E. coli protein extracts lacking His6-HuR were used as a negative control (lane 1). (B) Coimmunoprecipitation of HuR with S-HDAg. Huh7-D12 cell lysates were immunoprecipitated with an anti-HDAg antibody bound to protein G beads. The immunoprecipitates were run separated on 12% SDS/PAGE gels and immunoblotted with an anti-HuR or anti-HDAg antibody. The negative controls were prepared in the absence of the anti-HDAg antibody or using Huh7 cell protein extracts.