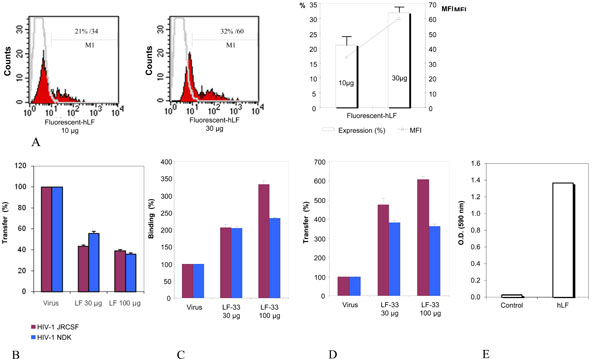
Fig. (5) Inhibition of HIV-1 transfer from iDC to CD4 T lymphocytes by hLF, not LF-33 peptide. (A) hLF binding to iDC. Cells (106 cells) were incubated with Oregon-conjugated hLF (10 and 30 µg, e.g. 128 and 384 nM) for 1 h at 4°C and analysed by FACScalibur. Data are from a typical experiment representative of four independent experiments. Histograms depict the mean ± SD of binding (%) and mean fluorescences obtained from 4 independent experiments. (B) Effect of hLF on HIV transfer from iDC to CD4 T lymphocytes. iDC were incubated or not with purified hLF (30 and 100 µg, e.g. 384 and 1280 nM) before addition of HIV-1 for 1 h. Cells were washed and cocultured for 3 days with autologous CD4 T lymphocytes. HIV p24 antigen was measured in supernatants by ELISA at day 3. Results are represented as percentage of the control (performed without pre-incubation of cells with hLF), and is the mean of transfer ± SD from 3 independent experiments. (C) Effect of LF-33 peptide on HIV-1 attachment to iDC. iDC were incubated with virus (5 ng/ml of p24 antigen of HIV-1JR-CSF and HIV-1NDK) for 1 h at 37°C. After several washes, cells were lysed and HIV p24 antigen was measured by ELISA. Cells were pre-incubated with the LF-33 peptide at 30 µg and 100 µg/ml (e.g. 7.5 µM and 25 µM) for 30 min at room temperature before virus addition. Results are represented as percentage of the control (performed without pre-incubation of cells with LF-33 peptide), and is the mean of binding ± SD from 3 independent experiments.As negative control, an irrelevant peptide was used instead LF-33 (not shown). (D) Effect of LF-33 peptide on HIV transfer from iDC to T cells. iDC were incubated with virus (5 ng/ml of p24 antigen of HIV-1JR-CSF and HIV-1NDK) for 1 h at 37°C. After several washes, IL-2-stimulated T cells were added to iDC and co-cultured for 72 h before p24 antigen measurement.As assessed by binding inhibition experiment, iDC were pre-incubated with the LF-33 peptide at 30 µg and 100 µg/ml (e.g. 7.5 µM and 25 µM) for 30 min at room temperature before virus addition. Results are represented as percentage of the control (performed without pre-incubation of cells with LF-33 peptide), and is the mean of transfer ± SD from 3 independent experiments. As negative control, an irrelevant peptide was used instead LF-33 (not shown). (E) Binding of LF to DC-SIGN. DC-SIGN “342-371” peptide was adsorbed (10 µg/ml) overnight at 4°C. Wells of ELISA plate were satured using PBS/1% powder milk for 2 h at 37°C before addition of purified hLF at 100 µg/ml overnight at 4°C. The fixed hLF was detected by peroxydase-conjugated polyclonal antibody to hLF (1 µg/ml). As a control, the same experiment was performed in parallel without DC-SIGN “342-371” peptide adsorption.