- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

Current Chemical Genomics and Translational Medicine
(Discontinued)
ISSN: 2213-9885 ― Volume 12, 2018
Identification of Thyroid Hormone Receptor Active Compounds Using a Quantitative High-Throughput Screening Platform
Jaime Freitas1, 5, Nicole Miller2, Brenda J. Mengeling3, Menghang Xia2, Ruili Huang2, Keith Houck4, Ivonne M.C.M. Rietjens1, J. David Furlow3, *, #, Albertinka J. Murk1, 6, #
Abstract
To adapt the use of GH3.TRE-Luc reporter gene cell line for a quantitative high-throughput screening (qHTS) platform, we miniaturized the reporter gene assay to a 1536-well plate format. 1280 chemicals from the Library of Pharmacologically Active Compounds (LOPAC) and the National Toxicology Program (NTP) 1408 compound collection were analyzed to identify potential thyroid hormone receptor (TR) agonists and antagonists. Of the 2688 compounds tested, eight scored as potential TR agonists when the positive hit cut-off was defined at ≥10% efficacy, relative to maximal triiodothyronine (T3) induction, and with only one of those compounds reaching ≥20% efficacy. One common class of compounds positive in the agonist assays were retinoids such as all-trans retinoic acid, which are likely acting via the retinoid-X receptor, the heterodimer partner with the TR. Five potential TR antagonists were identified, including the antiallergy drug tranilast and the anxiolytic drug SB 205384 but also some cytotoxic compounds like 5-fluorouracil. None of the inactive compounds were structurally related to T3, nor had been reported elsewhere to be thyroid hormone disruptors, so false negatives were not detected. None of the low potency (>100µM) TR agonists resembled T3 or T4, thus these may not bind directly in the ligand-binding pocket of the receptor. For TR agonists, in the qHTS, a hit cut-off of ≥20% efficacy at 100 µM may avoid identification of positives with low or no physiological relevance. The miniaturized GH3.TRE-Luc assay offers a promising addition to the in vitro test battery for endocrine disruption, and given the low percentage of compounds testing positive, its high-throughput nature is an important advantage for future toxicological screening.
Article Information
Identifiers and Pagination:
Year: 2014Volume: 8
First Page: 36
Last Page: 46
Publisher Id: CCGTM-8-36
DOI: 10.2174/2213988501408010036
Article History:
Received Date: 2/11/2013Revision Received Date: 9/12/2013
Acceptance Date: 12/12/2013
Electronic publication date: 7/3/2014
Collection year: 2014
open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
* Address correspondence to this author at the Department of Neurobiology, Physiology, and Behavior, University of California, Davis, CA 95616; Tel: (530)754-8609; Fax: (530) 752-5582. E-mail: jdfurlow@ucdavis.edu# Both authors contributes equally.
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 2-11-2013 |
Original Manuscript | Identification of Thyroid Hormone Receptor Active Compounds Using a Quantitative High-Throughput Screening Platform | |
INTRODUCTION
The vertebrate thyroid hormone (TH) system isan elaborate signaling network that controls critical processes through different life-stages,such as regulation of fuel metabolism [1Cheng SY, Leonard JL, Davis PJ. Molecular aspects of thyroid hormone actions Endocr Rev 2010; 31(2): 139-70.
[http://dx.doi.org/10.1210/er.2009-0007] ], proliferation versusdifferentiation, development and maintenance of brain function [2Bernal J. Thyroid hormone receptors in brain development and function Nat Clin Pract Endocrinol Metab 2007; 3(3): 249-59.
[http://dx.doi.org/10.1038/ncpendmet0424] -4Warner A, Mittag J. Thyroid hormone and the central control of homeostasis J Mol Endocrinol 2012; 49(1): R29-35.
[http://dx.doi.org/10.1530/JME-12-0068] ], thermoregulation [5Ribeiro MO. Effects of thyroid hormone analogs on lipid metabolism and thermogenesis Thyroid 2008; 18(2): 197-203.
[http://dx.doi.org/10.1089/thy.2007.0288] ], osmoregulation and renal function [6Vargas F, Moreno JM, Rodriguez-Gomez I, et al. Vascular and renal function in experimental thyroid disorders Eur J Endocrinol 2006; 154(2): 197-212.
[http://dx.doi.org/10.1530/eje.1.02093] ], seasonal reproductive behaviour and fertility [7Yoshimura T. Neuroendocrine mechanism of seasonal reproduction in birds and mammals Anim Sci J 2010; 81(4): 403-10.
[http://dx.doi.org/10.1111/j.1740-0929.2010.00777.x] , 8Wagner MS, Wajner SM, Maia AL. The role of thyroid hormone in testicular development and function J Endocrinol 2008; 199(3): 351-65.
[http://dx.doi.org/10.1677/JOE-08-0218] ], cardiovascular function [9Danzi S, Klein I. Cardiac specific effects of thyroid hormone analogues Horm Metab Res 2011; 43(11): 737-42.
[http://dx.doi.org/10.1055/s-0031-1291177] , 10Kahaly GJ, Dillmann WH. Thyroid hormone action in the heart Endocr Rev 2005; 26(5): 704-28.
[http://dx.doi.org/10.1210/er.2003-0033] ], and special senses [11Forrest D, Swaroop A. Minireview: the role of nuclear receptors in photoreceptor differentiation and disease Mol Endocrinol 2012; 26(6): 905-15.
[http://dx.doi.org/10.1210/me.2012-1010] ]. Potential alterations to the TH system by natural or synthetic compounds present in our food or the environment could therefore have substantial implications. In this context, the demonstration that certain manufactured chemicals exhibit thyroid hormone-like activity [12Gray LE Jr, Ostby J, Marshall R, et al. Reproductive and thyroid effects of low-level polychlorinated biphenyl (Aroclor 1254) exposure Fundam Appl Toxicol 1993; 20(3): 288-94.
[http://dx.doi.org/10.1006/faat.1993.1038] , 13Porterfield SP, Hendry LB. Impact of PCBs on thyroid hormone directed brain development Toxicol Ind Health 1998; 14(1-2): 103-20.
[http://dx.doi.org/10.1177/074823379801400109] ] generated an interest in the many potential adverse outcomes of TH system disruption. The TH signaling network relies on efficient and accurate interpretation of these extracellular chemical signals by the thyroid receptors (TRs) [1Cheng SY, Leonard JL, Davis PJ. Molecular aspects of thyroid hormone actions Endocr Rev 2010; 31(2): 139-70.
[http://dx.doi.org/10.1210/er.2009-0007] ]. Thyroid active compounds may interact at the level of these TRs but may also generate effects at several other targets in a normal functioning TH endocrine system. These include TH transport by transthyretin (TTR) or thyroxine-binding globulin (TBG) [14Lam M, Klasson CE, Wehler M, et al. Structure-dependent,
competitive interaction of hydroxy-polychlorobiphenyls, -dibenzo-p-dioxins and -dibenzofurans with human transthyretin Chem Biol Interact 1993; 88(1): 7-21.
[http://dx.doi.org/10.1016/0009-2797(93)90081-9] ]; hormone production which depends on iodine uptake [15Hornung MW, Degitz SJ, Korte LM, et al. Inhibition of thyroid hormone release from cultured amphibian thyroid glands by methimazole, 6-propylthiouracil, and perchlorate Toxicol Sci 2010; 118(1): 42-51.
[http://dx.doi.org/10.1093/toxsci/kfq166] ] and the enzyme thyroid peroxidase (TPO) to incorporate iodine onto tyrosinesof thyroglobulin [16Biegel LB, Cook JC, O'Connor JC, et al. Subchronic toxicity study in rats with 1-methyl-3-propylimidazole-2-thione (PTI): effects on the thyroid Fundam Appl Toxicol 1995; 27(2): 185-94.
[http://dx.doi.org/10.1006/faat.1995.1123] ]; hormone activation or deactivation by iodothyronine deiodinases types I, II, and III (D1, D2, and D3, respectively), which regulate the activity of thyroid hormones via removal of specific iodine substituents [17Darras VM, Van Herck SL. Iodothyronine deiodinase structure and function: from ascidians to humans J Endocrinol 2012; 215(2): 189-206.
[http://dx.doi.org/10.1530/JOE-12-0204] , 18Visser TJ, van Overmeeren E, Fekkes D, et al. Inhibition of iodothyronine 5'-deiodinase by thioureylenes; structure--activity relationship FEBS Lett 1979; 103(2): 314-8.
[http://dx.doi.org/10.1016/0014-5793(79)81352-6] ]; UDP glucuronosyltransferases (UGTs) or sulfotransferases (SULTs) which conjugate the thyroid hormones and facilitate their excretion from the body [19Schuur AG, Legger FF, van Meeteren ME, et al. In vitro inhibition of thyroid hormone sulfation by hydroxylated metabolites of halogenated aromatic hydrocarbons Chem Res Toxicol 1998; 11(9): 1075-81.
[http://dx.doi.org/10.1021/tx9800046] ]; or transporters of thyroid hormones through the target cell membrane [20Guo GL, Choudhuri S, Klaassen CD. Induction profile of rat organic anion transporting polypeptide 2 (oatp2) by prototypical drug-metabolizing enzyme inducers that activate gene expression through ligand-activated transcription factor pathways J Pharmacol Exp Ther 2002; 300(1): 206-12.
[http://dx.doi.org/10.1124/jpet.300.1.206] ]. Although in vitro assays have already been developed for several of these targets [21Murk AJ, Rijntjes E, Blaauboer BJ, et al. Mechanism-based testing strategy using in vitro approaches for identification of thyroid hormone disrupting chemicals Toxicol In Vitro 2013; 27(4): 1320-46.
[http://dx.doi.org/10.1016/j.tiv.2013.02.012] ], current risk assessment strategies still rely heavily on chemical safety data obtained in animal models. This low-throughput approach is relatively expensive and may provide an unreliable representation of human toxicity. Furthermore, the use of large numbers of animals for toxicity testing raises legal and ethical considerations. The development of integrated and intelligent testing strategies for toxicity evaluation, such as innovative in vitro and in silico approaches, has paved the way for the reduction of vertebrate studies. The regulatory system for chemicals controlled by the European Chemical Agency (ECHA), called Registration, Evaluation and Authorization of Chemicals (REACH) has placed a premium on functional, quantitative, high-throughput, in vitro screening assays (qHTS) for the toxicological evaluation of the extraordinarily high number of natural and synthetic chemicals to be assessed within a few years (about 30,000 substances are currently marketed at volumes greater than 1 ton/year). In addition, a collaboration known as Tox21, comprised of the United States Environmental Protection Agency (US EPA), the US National Institutes of Health (NIH), and the US Food and Drug Regulatory Agency (FDA), has initiated a program of screening a large chemical library composed of environmental chemicals and pharmaceuticals through different qHTS assays developed based on specific biological mechanisms relevant to toxicity [22Shukla SJ, Huang R, Austin CP, Xia M. The future of toxicity testing: a focus on in vitro methods using a quantitative high-throughput screening platform Drug Discov Today 2010; 15(23-24): 997-1007.
[http://dx.doi.org/10.1016/j.drudis.2010.07.007] -24Kavlock RJ, Austin CP, Tice RR. Toxicity testing in the 21st century: implications for human health risk assessment Risk Anal 2009; 29(4): 485-7. discussion 492-7
[http://dx.doi.org/10.1111/j.1539-6924.2008.01168.x] ]. These screening assays directly assess the effects of thousands of chemicals on particular cellular systems or molecular targets. However, for TR-mediated disruption, a functional qHTS assay based on endogenous full-length receptors is still lacking. Recently, we have developed and validated a stably-transfected reporter gene cellular model in the TH-responsive rat pituitary tumor GH3 cell line that endogenously expresses both TR isoforms [25Freitas J, Cano P, Craig-Veit C, et al. Detection of thyroid hormone receptor disruptors by a novel stable in vitro reporter gene assay Toxicol In Vitro 2011; 25(1): 257-66.
[http://dx.doi.org/10.1016/j.tiv.2010.08.013] ]. Here, we present the development and application of the GH3.TRE-luc cell line using a qHTS system in order to rapidly identify chemicals that alter TR activity, and therefore havethe potential for endocrine disruption. We miniaturized and optimized the GH3.TRE-Luc assay into a 1536-wells plate format for assaying potential agonistic, antagonistic and cytotoxic activities of the compounds tested. We used the optimized qHTS system to test the 1280 compounds of the LOPAC library (Library of Pharmacologically Active Compounds) [26Xia M, Guo V, Huang R, et al. Inhibition of morphine-induced cAMP overshoot: a cell-based assay model in a high-throughput format Cell Mol Neurobiol 2011; 31(6): 901-7.
[http://dx.doi.org/10.1007/s10571-011-9689-y] ] and the 1408 chemicals from the National Toxicology Program collection (NTP) [27Xia M, Huang R, Kristine LW, et al. Compound cytotoxicity profiling using quantitative high-throughput screening Environ Health Perspect 2008; 116(3): 284-91.
[http://dx.doi.org/10.1289/ehp.10727] ]. To insure that observed effects were not due to cytotoxicity, we measured intracellular ATP content as a cell viability readout. These chemical collections were used for validation of the high-throughput screen (qHTS) because of the diverse chemical families they contain, some of which are proven to be pharmacologically active, and with almost all the compounds previously tested in one or more standard toxicological assays. The outcomes of this preliminary screen were further examined to identify potential false positives and false negatives using the publically available PubChem Bioassay database.
MATERIALS AND METHODS
Cell Line and Culture Conditions
The GH3.TRE-Luc cell line, developed as described [25Freitas J, Cano P, Craig-Veit C, et al. Detection of thyroid hormone receptor disruptors by a novel stable in vitro reporter gene assay Toxicol In Vitro 2011; 25(1): 257-66.
[http://dx.doi.org/10.1016/j.tiv.2010.08.013] ], stably expresses a modified firefly luciferase reporter gene under the regulation of a pair of thyroid hormone response elements (TREs). Cells were routinely sub-cultured once a week in fresh 75-cm2 culture flasks (Corning, Acton, MA), in a humid atmosphere at 37°C and 95% air/5% CO2 in Dulbecco’s Modified Eagle’s medium/Ham’s F12 (DMEM:F12, Invitrogen, Carlsbad, CA) supplemented with 10% Fetal Bovine Serum (FBS, Hyclone, Logan, UT).
qHTS TRE Luciferase Reporter Gene Assay
For the TRE luciferase reporter gene assay GH3.TRE-Luc cells were seeded at 80% confluence in 225 cm2 culture flasks (Corning, Acton, MA) in regular growth medium and cultured overnight. Growth medium was then replaced by assay medium [28Sirbasku DA, Pakala R, Sato H, Eby JE. Thyroid hormone
dependent pituitary tumor cell growth in serum-free chemically
defined culture. A new regulatory role for apotransferrin Biochemistry 1991; 30(30): 7466-7.
[http://dx.doi.org/10.1021/bi00244a015] ] (DMEM:F12 supplemented with 10 µg/ml insulin, 10 µM ethanolamine, 10 ng/ml sodium selenite, 10 µg/ml human apotransferrin and 500 µg/ml bovine serum albumin) followed by a 20h incubation. Subsequently, cells were detached, re-suspended in assay medium. For agonist mode screening, cells were dispensed at 1500 cells/ 5 µl/well in 1536-well white wall/solid bottom plates (Greiner Bio-One North America, NC, USA) using a Flying Reagent Dispenser (FRD) (Aurora Discovery, CA, USA). After the cells were incubated at 37°C for 4-5 h, 23 nl of compounds at 7 to 14concentrations or DMSO control were transferred using a Pintoolstation (Wako, San Diego, CA) into each well resulting in a final DMSO concentration of 0.46 or 0.92%. For antagonist mode screening, cells were dispensed at 1500 cells/ 4 µl/wellin 1536-well white wall/solid bottom plates (Greiner Bio-One North America) using of FRD (Aurora Discovery). After test compounds were added as indicated above, 1 µl of 1 nM final concentration T3 or assay media control was dispensed to each well. The assay plates were incubated with compound treatment for 24h. After this incubation, 5 µl of One-Glo luciferase reagent (Promega, Madison, WI) was added and plates were incubated at room temperature for 30 min before reading on a Viewlux plate reader (PerkinElmer, Waltham, MA).
For the primary screening, the 1280 compounds from the Library of Pharmacologically Active Compounds (LOPAC, Sigma, St. Louis, Missouri, USA) and the 1408 chemicals from the National Toxicology Program collection (NTP-1408) [27Xia M, Huang R, Kristine LW, et al. Compound cytotoxicity profiling using quantitative high-throughput screening Environ Health Perspect 2008; 116(3): 284-91.
[http://dx.doi.org/10.1289/ehp.10727] ] were tested in series of 7 to 14 dilutions with final concentrations ranging from 0.6 nM to 92 µM and 3 nM to 46 µM for the NTP and LOPAC collections, respectively. The highest concentrations tested were judged to be at the very upper range of potential environmental exposures. The four left columns in each plate were reserved for controls. The control format for the agonist mode plate was column-1 T3 from 0.3 pM to 4.6 µM, column-2 100 nMT3 and column 3 to 4 DMSO only. The control format for the antagonist mode plate was column-1 to 2 DMSO only, column-3 to 48 1 nMT3.
For rescreening and testing nuclear receptor specific ligands, GH3.TRE-Luc cells were seeded at 150,000 cells/well in 24-well tissue culture plates (Greiner Bio One, Monroe, NC) in DMEM:F12 with 15 mM HEPES (Gibco-Invitrogen, Grand Island, NY) supplemented with 10% fetal bovine serum (Gibco-Invitrogen) and Pen/Strep (Gibco-Invitrogen). Twenty-four hours later, growth medium was replaced by PCM medium for an additional 24h. Cells were then incubated for 24h in the presence or absence of the indicated ligands in 0.2 % DMSO. Cell numbers were estimated using a BCA protein assay (Thermo Scientific, Rockford, IL) and luciferase activity was measured from lysed cells in a Hidex Chameleon V microplate luminometer using the Luciferase Assay System (Promega, Madison, WI). Each dose within an experiment was treated in duplicate, and each experiment was performed at least three times.
Cell Viability Assay
In order to exclude the compounds that inhibit TR-induced luciferase reporter gene expression due to cytotoxicity, the LOPAC and NTP libraries were also tested for cell viability by measuring intracellular ATP content using a luciferase-coupled ATP quantitation assay (CellTiter-Glo viability assay, Promega, Madison, WI). The cells were dispensed at 1500 cells/5 µl/well in 1536-well white/solid bottom assay plates (GreinerBio-One North America) and the assay was run identically to the antagonist screen method mentioned above, with addition of 5 µl/well of CellTiter-Glo reagent in place of One-Glo. After 30 minutes incubation at room temperature, the luminescence intensity was measured using a ViewLux plate reader (PerkinElmer).
Data Analysis
The primary data analysis was performed as previously described. Briefly, raw plate reads for each titration point were first normalized relative to the T3 control (100 nM T3, set at 100% for agonist mode; 1 nM T3, set at 0% for antagonist mode) and DMSO only wells (basal, set at 0% for agonist mode and -100% for antagonist mode), and then corrected by applying a pattern correction algorithm using compound-free control plates (DMSO plates) [29Xia M, Shahane SA, Huang R, et al. Identification of quaternary ammonium compounds as potent inhibitors of hERG potassium channels Toxicol Appl Pharmacol 2011; 252(3): 250-8.
[http://dx.doi.org/10.1016/j.taap.2011.02.016] ].
Concentration-response titration points for each compound were fitted to the Hill equation yielding concentrations for half-maximal activity for agonists (EC50) or half-maximal inhibition for antagonists (IC50) and maximal response (efficacy) values. The concentration response curves of the compounds were classified into four major classes (1-4) based on the completeness of curve, goodness of fit, and efficacy [29Xia M, Shahane SA, Huang R, et al. Identification of quaternary ammonium compounds as potent inhibitors of hERG potassium channels Toxicol Appl Pharmacol 2011; 252(3): 250-8.
[http://dx.doi.org/10.1016/j.taap.2011.02.016] ]. Antagonists were identified using the selection criterion that their IC50 values should be at least three times lower than the IC50 in the viability assay to exclude cases of cytotoxicity.
RESULTS
Assay Optimization and Miniaturization in the 1536-Well Plate Format
The GH3.TRE-luc assay was initially miniaturized in a 1536-well plate with a 5 µl final assay volume. To find the optimal cell density for the well, three different cell numbers were tested after treatment with various concentrations of the known agonist, T3, ranging from 0.3 pM to 4.6 uM for 24 h. The EC50 values of T3 obtained were 0.33, 0.55, and 0.39 nM at cell densities of 1000, 1500, and 2000 cells/well, respectively (Table 1). The signal-to-background ratio (S/B) for these three cell densities was 7 to 9.5 fold. Z’ factor value from the density of 1500 cells/well was 0.88, which was the highest compared to other cell densities (1000 or 2000 cells/well). Therefore, we chose 1500 cells/well for use in subsequent studies.
We optimized conditions to screen for thyroid receptor antagonist activity. Antagonist action can be identified based on the ability of the test compound to block the effect of a sub-maximal concentration of the agonist T3. In order to determine the concentration of T3 to be used for qHTS determination of antagonist activity, two concentrations slightly above the EC50 of T3 (Table 2) were tested. At both T3 concentrations the assay showed a similar S/B ratio (3.6 and 3.7 fold) but the 1 nM T3 group gave a minimal CV (coefficient of variation, 7%) compared to the 0.5 nM T3 group (CV, 11%). Z factors [30Zhang JH, Chung TD, Oldenburg KR. A simple statistical parameter for use in evaluation and validation of high throughput screening assays J Biomol Screen 1999; 4(2): 67-73.
[http://dx.doi.org/10.1177/108705719900400206] ] were 0.44 and 0.65 for the 0.5 nM and 1 nM T3 exposure groups, indicating that 1 nM was a better concentration for screening for antagonist activity. This concentration represents one that optimizes a large screening window without significant loss of sensitivity to detecting antagonists due to receptor binding competition with the T3. All further inhibition (antagonism) assays were run using 1 nM of T3 as the agonist.
Identification of Potential TR Agonists by qHTS
The qHTS GH3.TRE-luc assay was used to screen the LOPAC and NTP libraries for TR agonists and antagonists to provide a proof of principle for its use as a newly developed biomolecular screening tool. Structures of representative compounds that showed positive responses in the qHTS assay are shown in Fig. (1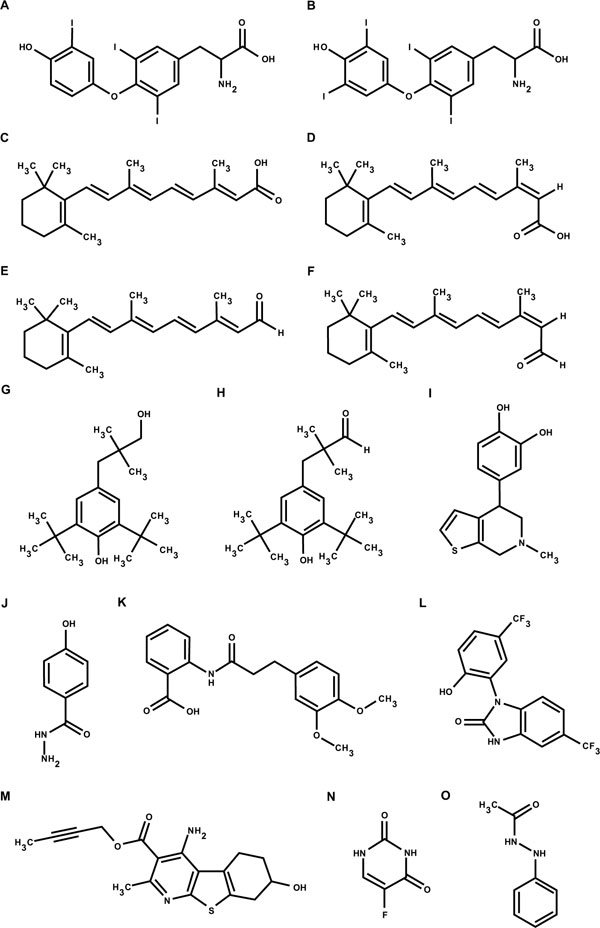 ). For agonist screening, the concentration titration of T3, used as a positive control, was performed in each plate. The control dose-response curves of T3 were well reproduced in all 27 plates used for the screening of the two libraries, including 6 DMSO plates, with an EC50 average of 0.66 ± 0.13 nM (Fig. 2
). For agonist screening, the concentration titration of T3, used as a positive control, was performed in each plate. The control dose-response curves of T3 were well reproduced in all 27 plates used for the screening of the two libraries, including 6 DMSO plates, with an EC50 average of 0.66 ± 0.13 nM (Fig. 2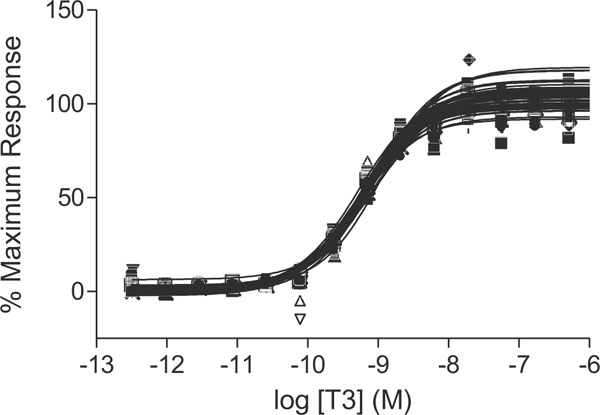 ). T3 controls averaged a signal-to-background ratio of 6.88 and 7.42 and the average Z factor was 0.82 and 0.77 in the LOPAC and NTP libraries screen, respectively (Table 3). Of the 1280 compounds from the LOPAC library, 6 (0.5%) were identified as potential TR agonists (Fig. 3
). T3 controls averaged a signal-to-background ratio of 6.88 and 7.42 and the average Z factor was 0.82 and 0.77 in the LOPAC and NTP libraries screen, respectively (Table 3). Of the 1280 compounds from the LOPAC library, 6 (0.5%) were identified as potential TR agonists (Fig. 3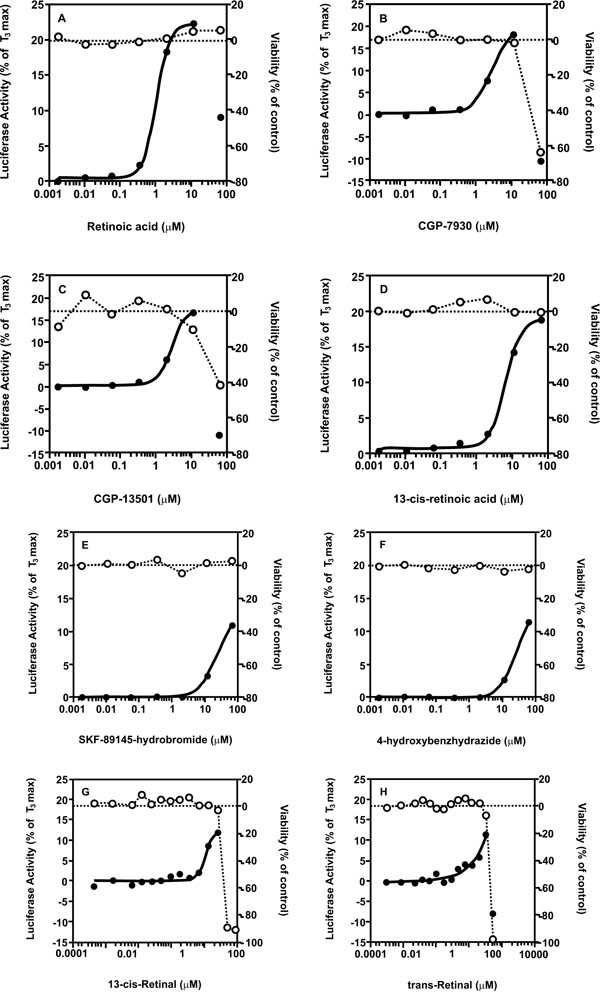 ), with the positive hit cut-off being compounds that gave a ≥10% efficacy. The potency and efficacy of these compounds are listed in Table 4. Of the 1408 compounds from the NTP library, 2 (0.1%) were identified as potential TR agonists, with the positive hit cut-off being compounds that gave a ≥10% efficacy. The potency and affinity of these compounds are listed in Table 5. In order to evaluate the reproducibility of the hits in the qHTS format, the LOPAC library was re-screened three times. All 6 compounds identified from the primary screen showed similar activity in the re-screen (Table 4). When, for defining TR agonist activity, the positive hit cut-off was set at ≥20% efficacy, only one TR agonist, retinoic acid, was identified in the LOPAC library and none in the NTP library. Close evaluation of the dose response curves for the compounds that did not induce ≥20% efficacy, revealed that at the highest dose levels tested, 46 or 92 µM, maximum agonist activity may not yet have been reached for some of the compounds. However, given that the highest concentrations tested were generally considered to be at the upper end of possible exposure levels, we conclude that those compounds may not represent effective TR agonists. Furthermore, none of these compounds has structural characteristics that resemble the thyroid hormones T3 and T4 (Fig. 1
), with the positive hit cut-off being compounds that gave a ≥10% efficacy. The potency and efficacy of these compounds are listed in Table 4. Of the 1408 compounds from the NTP library, 2 (0.1%) were identified as potential TR agonists, with the positive hit cut-off being compounds that gave a ≥10% efficacy. The potency and affinity of these compounds are listed in Table 5. In order to evaluate the reproducibility of the hits in the qHTS format, the LOPAC library was re-screened three times. All 6 compounds identified from the primary screen showed similar activity in the re-screen (Table 4). When, for defining TR agonist activity, the positive hit cut-off was set at ≥20% efficacy, only one TR agonist, retinoic acid, was identified in the LOPAC library and none in the NTP library. Close evaluation of the dose response curves for the compounds that did not induce ≥20% efficacy, revealed that at the highest dose levels tested, 46 or 92 µM, maximum agonist activity may not yet have been reached for some of the compounds. However, given that the highest concentrations tested were generally considered to be at the upper end of possible exposure levels, we conclude that those compounds may not represent effective TR agonists. Furthermore, none of these compounds has structural characteristics that resemble the thyroid hormones T3 and T4 (Fig. 1 ), providing additional support for the conclusion that they may not represent effective direct TR agonists.
), providing additional support for the conclusion that they may not represent effective direct TR agonists.
Retinoids detected as positives in the qHTS assay are likely acting as agonists via RXRs and not RARs, or RXRs complexed with permissive liver –X receptors (LXRs)
In both the LOPAC and NTP1408 libraries, Vitamin A derivatives (retinoids) scored as positive hits in the agonist mode assay. This raised the possibility that in addition to the TRs, the system was responsive to retinoic acid receptors (RARs) or retinoid X receptors (RXRs), either alone or in known permissive heterodimer complexes with other nuclear receptors such as the liver X receptors (LXRs) [31Repa JJ, Turley SD, Lobaccaro JA, et al. Regulation of absorption and ABC1-mediated efflux of cholesterol by RXR heterodimers Science 2000; 289(5484): 1524-9.
[http://dx.doi.org/10.1126/science.289.5484.1524] ]. However, neither the RAR-specific ligand TTNPB nor the LXR-specific ligand T0901317 activated the reporter gene, under conditions where both T3 and all-trans retinoic acid showed the expected induction profiles (Fig. 4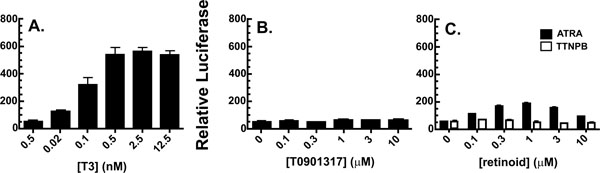 ).
).
Identification of Potential TR Antagonists by qHTS
To identify potential TR antagonists using this GH3.TRE-luc assay, the LOPAC and NTP libraries were screened in the antagonist mode, where cells were exposed to the test compounds in the presence of 1 nM T3, and the ability of the compounds to inhibit T3-mediated TR activation was measured. The average signal-to-background ratio was 3.37 and 4.71, and the average Z factor was 0.76 and 0.65 for the LOPAC and NTP libraries, respectively. To ascertain that the inhibitory effect of the potential hits was not due to cytotoxic effects, a cell viability screening was performed in parallel. Compounds that are known inhibitors of luciferase activity or expression, such as cycloheximide that is a potent translation inhibitor present in the LOPAC library, were removed from further consideration as an additional filter. From the LOPAC screen, 4 compounds (0.3%) were identified with an IC50 that was 3-fold lower than their viability IC50. The potency and efficacy of these compounds are listed in Table 4 and graphs are given in Fig. (5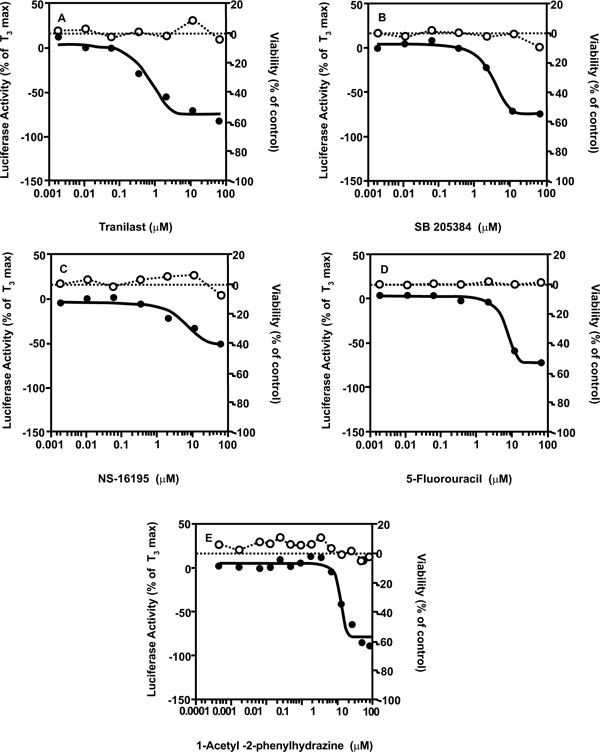 ). From the NTP library, 2 compounds (0.1%) were identified using the same selection criteria. The potency and efficacy of these compounds are listed in Table 5 and graphs are given in Fig. (5
). From the NTP library, 2 compounds (0.1%) were identified using the same selection criteria. The potency and efficacy of these compounds are listed in Table 5 and graphs are given in Fig. (5 ). In order to evaluate the reproducibility of the hits in the qHTS format, the LOPAC library was also screened an additional three times. All 4 compounds of interest were confirmed in the re-screening assays (Table 4).
). In order to evaluate the reproducibility of the hits in the qHTS format, the LOPAC library was also screened an additional three times. All 4 compounds of interest were confirmed in the re-screening assays (Table 4).
Altogether the data presented indicate reproducibility and provide a first proof of principle that the GH3.TRE-luc assay is robust and can be utilized to screen large compound libraries in the 1536- well plate format for potential TR agonist as well as antagonist activity.
DISCUSSION
In the present study, we developed a quantitative high-throughput screen (qHTS) for potential TR agonist and antagonist activity that was based upon the recently developed in vitro reporter gene assay using the stably transfected GH3.TRE-Luc cell line [25Freitas J, Cano P, Craig-Veit C, et al. Detection of thyroid hormone receptor disruptors by a novel stable in vitro reporter gene assay Toxicol In Vitro 2011; 25(1): 257-66.
[http://dx.doi.org/10.1016/j.tiv.2010.08.013] ]. The assay was miniaturized and validated in a 1536-well plate format. Subsequently the 1280 compounds of the LOPAC library and the 1408 compounds of the NTP collection were tested for their TR agonist or antagonist activity. Of the 2688 compounds tested in the qHTS 8 (0.3%) or 1 (0.04%) were found to be TR agonists depending on whether the positive hit cut off was defined at ≥10% or ≥20% efficacy. None of the inactive compounds was structurally related to T3, nor had been reported elsewhere to be a thyroid hormone disruptor, so false negatives do not appear to be present in the screen. Furthermore, none of the low potency TR agonists had structural characteristics that resemble the thyroid hormones (Fig. 1 ), providing further support that they may not represent effective,direct TR agonists, and therefore may not activate the luciferase gene through direct binding to the TR ligand-binding pocket. Defining TR agonists in the qHTS with a hit cut off of ≥20% efficacy at 100 μM may avoid identification of positives that are only very weak agonists and/or not likely to be acting through the ligand-binding pocket. Overall, the GH3.TRE-Luc cells performed very well in an automated 1536-well plate format. The CV slightly varied between 7-11%, but it was well within the performance standards for comparable assays in 1536-well plate format [27Xia M, Huang R, Kristine LW, et al. Compound cytotoxicity profiling using quantitative high-throughput screening Environ Health Perspect 2008; 116(3): 284-91.
), providing further support that they may not represent effective,direct TR agonists, and therefore may not activate the luciferase gene through direct binding to the TR ligand-binding pocket. Defining TR agonists in the qHTS with a hit cut off of ≥20% efficacy at 100 μM may avoid identification of positives that are only very weak agonists and/or not likely to be acting through the ligand-binding pocket. Overall, the GH3.TRE-Luc cells performed very well in an automated 1536-well plate format. The CV slightly varied between 7-11%, but it was well within the performance standards for comparable assays in 1536-well plate format [27Xia M, Huang R, Kristine LW, et al. Compound cytotoxicity profiling using quantitative high-throughput screening Environ Health Perspect 2008; 116(3): 284-91.
[http://dx.doi.org/10.1289/ehp.10727] ].
We used these two chemical collections to validate the highthroughput screen (HTS) because of their diverse chemical spaces, containing compounds proven to be pharmacologically active, and with almost all compounds previously tested in one or more standard toxicological assays. They do not, however, specifically contain compounds known for their in vivo or in vitro thyroid hormone disrupting potency. Not included in the assays were the known T3- and T4-like compounds, Tetrac (3,5,3’,5’-tetraiodothyroacetic acid) and Triac (3,5,3’-triiodothyroacetic acid),or any OH-PCBs (hydroxylated polychlorinated biphenyls), or PBDEs (polybrominated diphenylethers), which had been shown previously to be active in the GH3.TRE-Luc assay [25Freitas J, Cano P, Craig-Veit C, et al. Detection of thyroid hormone receptor disruptors by a novel stable in vitro reporter gene assay Toxicol In Vitro 2011; 25(1): 257-66.
[http://dx.doi.org/10.1016/j.tiv.2010.08.013] ]. The LOPAC library contains pharmacologically active compounds, including many cytostatic compounds that could cause growth inhibition thereby reducing luciferase activity. For compounds that do not directly antagonize TR activity, cell viability and TRE-Luc inhibition IC50’s will be similar. Therefore, it is important to quantify cell viability and to calculate the ratio between cell viability and the TRE-Luc inhibition IC50s. When this ratio is less than 3, we assumed the compound to be merely cytotoxic instead of potentially antagonistic of TR activity; this approach has been used before for other reporter gene screens [32Miller SC, Huang R, Sakamuru S, et al. Identification of known drugs that act as inhibitors of NF-kappaB signaling and their mechanism of action Biochem Pharmacol 2010; 79(9): 1272-80.
[http://dx.doi.org/10.1016/j.bcp.2009.12.021] ]. One of the most potent TRE-Luc antagonists identified in the present screen was the anti-cancer drug 5’-fluorouracil, inducing a full concentration-response curve without apparent cytotoxicity over the range of concentrations used within the 24 h treatment (Fig. 5 ). For known cytotoxic compounds like this, it would be worthwhile to evaluate cytotoxicity by alternative methods to provide higher confidence in interpretation of these findings. It is of interest to note that one of the side effects of 5-fluorouracil is cardiotoxicity, the mechanism of which is still poorly understood [33McGlinchey PG, Webb ST, Campbell NP. 5-fluorouracil-induced cardiotoxicity mimicking myocardial infarction: a case report BMC Cardiovasc Disord 2001; 1: 3.
). For known cytotoxic compounds like this, it would be worthwhile to evaluate cytotoxicity by alternative methods to provide higher confidence in interpretation of these findings. It is of interest to note that one of the side effects of 5-fluorouracil is cardiotoxicity, the mechanism of which is still poorly understood [33McGlinchey PG, Webb ST, Campbell NP. 5-fluorouracil-induced cardiotoxicity mimicking myocardial infarction: a case report BMC Cardiovasc Disord 2001; 1: 3.
[http://dx.doi.org/10.1186/1471-2261-1-3] ] but has not been linked to TR action to date. In addition, compounds like 5’-fluorouracil induce p53, which in turn is known to interact with and inhibit TR function [34Bhat MK, Yu Cl, Yap N, et al. Tumor suppressor p53 is a negative regulator in thyroid hormone receptor signaling pathways J Biol Chem 1997; 272(46): 28989-93.
[http://dx.doi.org/10.1074/jbc.272.46.28989] , 35Yap N, Yu CL, Cheng SY. Modulation of the transcriptional activity of thyroid hormone receptors by the tumor suppressor p53 Proc Natl Acad Sci USA 1996; 93(9): 4273-7.
[http://dx.doi.org/10.1073/pnas.93.9.4273] ]. Other potential modes of action of antagonistic compounds are possible that do not require direct TR binding, including inhibition of hormone uptake, induction of hormone export, induction of Type III deiodinase, or inhibition of TR coactivator expression or enzymatic activity. As the cells are used in larger chemicals screens, antagonism via a spectrum of TH- and TR-associated activities versus inhibition of luciferase activity or cell viability will be important to discern in secondary screens.
The most active agonists with EC50values lower than 10 μM were retinoids detected in both libraries (retinoic acid, trans-retinal, 13-cis-retinoic acid and 13-cis-retinal), and the positive allosteric modulators of the GABA receptors GCP-7930 and GCP-13501 (Fig. 3 ). GABA itself was present in the LOPAC library but did not show any response in this assay, meaning that the GCP compounds may not be activating the reporter gene via the GABA receptor. Retinoic acid is a known direct agonist of both the RAR and the TR heterodimer partner RXR, and the other retinoids may be converted to RAR and/or RXR agonists by intracellular metabolism and isomerization [36Blaner WS. Cellular metabolism and actions of 13-cis-retinoic acid J Am Acad Dermatol 2001; 45(5): S129-35.
). GABA itself was present in the LOPAC library but did not show any response in this assay, meaning that the GCP compounds may not be activating the reporter gene via the GABA receptor. Retinoic acid is a known direct agonist of both the RAR and the TR heterodimer partner RXR, and the other retinoids may be converted to RAR and/or RXR agonists by intracellular metabolism and isomerization [36Blaner WS. Cellular metabolism and actions of 13-cis-retinoic acid J Am Acad Dermatol 2001; 45(5): S129-35.
[http://dx.doi.org/10.1067/mjd.2001.113714] ]. However, RXRs are generally thought to form “nonpermissive” heterodimers with TRs, RARs,and Vitamin D receptors meaning that RXR ligands are not thought to be able to bind and activate in those complexes. Other nuclear receptors like peroxisome proliferator activated receptors (PPARs) or liver X receptors (LXRs) form “permissive” heterodimers with RXRs that can be activated by agonists that bind to either partner. In addition, RARs can heterodimerize with TRs and activate the DR4 elements as used in this assay, to some degree in transient transfection assays, in addition to their more preferred DR2 or DR5 arrangements that promote silent, nonpermissive complexes with RXRs [37Lee S, Privalsky ML. Heterodimers of retinoic acid receptors and thyroid hormone receptors display unique combinatorial regulatory properties Mol Endocrinol 2005; 19(4): 863-78.
[http://dx.doi.org/10.1210/me.2004-0210] ]. Furthermore, RXR/LXR heterodimers preferentially transactivate DR4 elements, and GH3 cells express functional RXRs [38Davis KD, Lazar MA. Selective antagonism of thyroid hormone action by retinoic acid J Biol Chem 1992; 267(5): 3185-9., 39Davis KD, Berrodin TJ, Stelmach JE, Winkler JD, Lazar MA. Endogenous retinoid X receptors can function as hormone receptors in pituitary cells Mol Cell Biol 1994; 14(11): 7105-10.], RARs [38Davis KD, Lazar MA. Selective antagonism of thyroid hormone action by retinoic acid J Biol Chem 1992; 267(5): 3185-9.], and LXRs [40Matsumoto S, Hashimoto K, Yamada M, Satoh T, Hirato J, Mori M. Liver X receptor-alpha regulates proopiomelanocortin (POMC) gene transcription in the pituitary Mol Endocrinol 2009; 23(1): 47-60.
[http://dx.doi.org/10.1210/me.2007-0533] ]. However, RARs and LXRs do not activate the reporter gene in these cells as judged by the lack of activity of specific synthetic ligands for these receptors. Thus, it appears likely that the retinoid activity is mediated by RXRs in these cells. Interestingly, the status of RXR as a permissive versus non-permissive partner is defined by the specific cell system used for evaluation. A series of previous studies demonstrated permissive actions of RXRs specifically in pituitary derived cells but not the other cell types [41Li D, Li T, Wang F, Tian H, Samuels HH. Functional evidence for retinoid X receptor (RXR) as a nonsilent partner in the thyroid hormone receptor/RXR heterodimer Mol Cell Biol 2002; 22(16): 5782-92.
[http://dx.doi.org/10.1128/MCB.22.16.5782-5792.2002] , 42Castillo AI, Sánchez-Martínez R, Moreno L, et al. A permissive retinoid X receptor/thyroid hormone receptor heterodimer allows stimulation of prolactin gene transcription by thyroid hormone and 9-cis-retinoic acid Mol Cell Biol 2004; 24(2): 502-13.
[http://dx.doi.org/10.1128/MCB.24.2.502-513.2004] ]. In these rat pituitary derived GH3 cells, RXR in the RXR:TR heterodimer may be permissively modulated by RXR ligands, and thus representing an important functional component of TR signaling in a cell type dependent manner. This would also explain the agonist-like behavior of retinoids in the GH3.TRE-Luc assay without any structural resemblance to T3 or T4. Comparison between the reporter gene activation in the GH3.TRE-Luc versusother cells where RXRs are not permissive in TR heterodimers could be used to filter out RXR-active compounds.
The reporter gene induction potency of other compounds that do not structurally resemble T3, is less than that of true TR-agonists such as the T3-like OH-BDEs [25Freitas J, Cano P, Craig-Veit C, et al. Detection of thyroid hormone receptor disruptors by a novel stable in vitro reporter gene assay Toxicol In Vitro 2011; 25(1): 257-66.
[http://dx.doi.org/10.1016/j.tiv.2010.08.013] ]. Therefore the cut-off of a maximum efficacy of ≥20% is also of use to filter out these compounds that are less likely to function as true TR agonists. These less effective activators could still be of interest as they may interact with pathways indirectly modulating TR activation, such as interactions with co-regulators or epigenetic modifications like effects on DNA methylation, chromatin structure or miRNA expression patterns, for example.
Important secondary assays in cell lines and in animals should follow to determine whether the GH3.TRE-LUC qHTS assay has good predictive power for effects on thyroid hormone signaling in vivo. For example, we are currently testing agonists and antagonists from this and subsequent larger screens against endogenous thyroid hormone responsive genes in the GH3.TRE-LUC cells. A number of TH regulated genes are expressed in GH3 cells, including the growth hormone gene that contains the first identified TRE [43Koenig RJ, Brent GA, Warne RL, Larsen PR, Moore DD. Thyroid hormone receptor binds to a site in the rat growth hormone promoter required for induction by thyroid hormone Proc Natl Acad Sci USA 1987; 84(16): 5670-4.
[http://dx.doi.org/10.1073/pnas.84.16.5670] ]. Compounds affecting endogenous genes are then prioritized for molecular mode of action studies (ligand binding, TRE binding, co activator or corepressor recruitment and other endpoints). Such prioritized compounds will then be tested further in vivo.We plan to first use the induced and spontaneous Xenopus laevis metamorphosis assays that we have used for a number of years to screen synthetic TR agonists and antagonists [44Furlow JD, Yang HY, Hsu M, et al. Induction of larval tissue resorption in Xenopus laevis tadpoles by the thyroid hormone receptor agonist GC-1 J Biol Chem 2004; 279(25): 26555-62.
[http://dx.doi.org/10.1074/jbc.M402847200] , 45Lim W, Nguyen NH, Yang HY, Scanlan TS, Furlow JD. A thyroid hormone antagonist that inhibits thyroid hormone action in vivo J Biol Chem 2002; 277(38): 35664-70.
[http://dx.doi.org/10.1074/jbc.M205608200] ], given the strong conservation of the TRs and other signaling components among vertebrates [46Furlow JD, Neff ES. A developmental switch induced by thyroid hormone: Xenopus laevis metamorphosis Trends Endocrinol Metab 2006; 17(2): 40-7.
[http://dx.doi.org/10.1016/j.tem.2006.01.007] ]. Certainly, studies in euthyroid and hypothyroid rodents, during critical windows of development and in the adult may follow for those compounds affecting endogenous genes in cell lines and tadpole development in vivo.
CONCLUSION
Taken together, the results obtained in the present study demonstrate the potential of the qHTS assay developed here for the identification of novel compounds acting via a TR-mediated mode of action. The miniaturized GH3.TRE-Luc assay offers a promising addition to the in vitro test battery for endocrine disruption, and that, given the low percentage of compounds testing positive, its high-throughput nature is an important advantage for future toxicological screening.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflicts of interest.
ACKNOWLEDGEMENTS
This research was financially supported by a ZonMW - NWO grant (11.400.0075) part of the Alternatives to Animal Experiments Program, and US EPA STAR grant 83516401to JFD and AJM. The Intramural Research Programs of Division of the National Toxicology Program, National Institute of Environmental Health Sciences, National Institutes of Health, as well as the U.S. Environmental Protection Agency also supported this work. The results and interpretations presented here are solely the authors and do not represent an official position of the U.S. EPA.
REFERENCES
| [1] | Cheng SY, Leonard JL, Davis PJ. Molecular aspects of thyroid hormone actions Endocr Rev 2010; 31(2): 139-70. [http://dx.doi.org/10.1210/er.2009-0007] |
| [2] | Bernal J. Thyroid hormone receptors in brain development and function Nat Clin Pract Endocrinol Metab 2007; 3(3): 249-59. [http://dx.doi.org/10.1038/ncpendmet0424] |
| [3] | Horn S, Heuer H. Thyroid hormone action during brain development: more questions than answers Mol Cell Endocrinol 2010; 315(1-2): 19-26. [http://dx.doi.org/10.1016/j.mce.2009.09.008] |
| [4] | Warner A, Mittag J. Thyroid hormone and the central control of homeostasis J Mol Endocrinol 2012; 49(1): R29-35. [http://dx.doi.org/10.1530/JME-12-0068] |
| [5] | Ribeiro MO. Effects of thyroid hormone analogs on lipid metabolism and thermogenesis Thyroid 2008; 18(2): 197-203. [http://dx.doi.org/10.1089/thy.2007.0288] |
| [6] | Vargas F, Moreno JM, Rodriguez-Gomez I, et al. Vascular and renal function in experimental thyroid disorders Eur J Endocrinol 2006; 154(2): 197-212. [http://dx.doi.org/10.1530/eje.1.02093] |
| [7] | Yoshimura T. Neuroendocrine mechanism of seasonal reproduction in birds and mammals Anim Sci J 2010; 81(4): 403-10. [http://dx.doi.org/10.1111/j.1740-0929.2010.00777.x] |
| [8] | Wagner MS, Wajner SM, Maia AL. The role of thyroid hormone in testicular development and function J Endocrinol 2008; 199(3): 351-65. [http://dx.doi.org/10.1677/JOE-08-0218] |
| [9] | Danzi S, Klein I. Cardiac specific effects of thyroid hormone analogues Horm Metab Res 2011; 43(11): 737-42. [http://dx.doi.org/10.1055/s-0031-1291177] |
| [10] | Kahaly GJ, Dillmann WH. Thyroid hormone action in the heart Endocr Rev 2005; 26(5): 704-28. [http://dx.doi.org/10.1210/er.2003-0033] |
| [11] | Forrest D, Swaroop A. Minireview: the role of nuclear receptors in photoreceptor differentiation and disease Mol Endocrinol 2012; 26(6): 905-15. [http://dx.doi.org/10.1210/me.2012-1010] |
| [12] | Gray LE Jr, Ostby J, Marshall R, et al. Reproductive and thyroid effects of low-level polychlorinated biphenyl (Aroclor 1254) exposure Fundam Appl Toxicol 1993; 20(3): 288-94. [http://dx.doi.org/10.1006/faat.1993.1038] |
| [13] | Porterfield SP, Hendry LB. Impact of PCBs on thyroid hormone directed brain development Toxicol Ind Health 1998; 14(1-2): 103-20. [http://dx.doi.org/10.1177/074823379801400109] |
| [14] | Lam M, Klasson CE, Wehler M, et al. Structure-dependent,
competitive interaction of hydroxy-polychlorobiphenyls, -dibenzo-p-dioxins and -dibenzofurans with human transthyretin Chem Biol Interact 1993; 88(1): 7-21. [http://dx.doi.org/10.1016/0009-2797(93)90081-9] |
| [15] | Hornung MW, Degitz SJ, Korte LM, et al. Inhibition of thyroid hormone release from cultured amphibian thyroid glands by methimazole, 6-propylthiouracil, and perchlorate Toxicol Sci 2010; 118(1): 42-51. [http://dx.doi.org/10.1093/toxsci/kfq166] |
| [16] | Biegel LB, Cook JC, O'Connor JC, et al. Subchronic toxicity study in rats with 1-methyl-3-propylimidazole-2-thione (PTI): effects on the thyroid Fundam Appl Toxicol 1995; 27(2): 185-94. [http://dx.doi.org/10.1006/faat.1995.1123] |
| [17] | Darras VM, Van Herck SL. Iodothyronine deiodinase structure and function: from ascidians to humans J Endocrinol 2012; 215(2): 189-206. [http://dx.doi.org/10.1530/JOE-12-0204] |
| [18] | Visser TJ, van Overmeeren E, Fekkes D, et al. Inhibition of iodothyronine 5'-deiodinase by thioureylenes; structure--activity relationship FEBS Lett 1979; 103(2): 314-8. [http://dx.doi.org/10.1016/0014-5793(79)81352-6] |
| [19] | Schuur AG, Legger FF, van Meeteren ME, et al. In vitro inhibition of thyroid hormone sulfation by hydroxylated metabolites of halogenated aromatic hydrocarbons Chem Res Toxicol 1998; 11(9): 1075-81. [http://dx.doi.org/10.1021/tx9800046] |
| [20] | Guo GL, Choudhuri S, Klaassen CD. Induction profile of rat organic anion transporting polypeptide 2 (oatp2) by prototypical drug-metabolizing enzyme inducers that activate gene expression through ligand-activated transcription factor pathways J Pharmacol Exp Ther 2002; 300(1): 206-12. [http://dx.doi.org/10.1124/jpet.300.1.206] |
| [21] | Murk AJ, Rijntjes E, Blaauboer BJ, et al. Mechanism-based testing strategy using in vitro approaches for identification of thyroid hormone disrupting chemicals Toxicol In Vitro 2013; 27(4): 1320-46. [http://dx.doi.org/10.1016/j.tiv.2013.02.012] |
| [22] | Shukla SJ, Huang R, Austin CP, Xia M. The future of toxicity testing: a focus on in vitro methods using a quantitative high-throughput screening platform Drug Discov Today 2010; 15(23-24): 997-1007. [http://dx.doi.org/10.1016/j.drudis.2010.07.007] |
| [23] | Huang R, Xia M, Cho MH, et al. Chemical genomics profiling of environmental chemical modulation of human nuclear receptors Environ Health Perspect 2011; 119(8): 1142-8. [http://dx.doi.org/10.1289/ehp.1002952] |
| [24] | Kavlock RJ, Austin CP, Tice RR. Toxicity testing in the 21st century: implications for human health risk assessment Risk Anal 2009; 29(4): 485-7. discussion 492-7 [http://dx.doi.org/10.1111/j.1539-6924.2008.01168.x] |
| [25] | Freitas J, Cano P, Craig-Veit C, et al. Detection of thyroid hormone receptor disruptors by a novel stable in vitro reporter gene assay Toxicol In Vitro 2011; 25(1): 257-66. [http://dx.doi.org/10.1016/j.tiv.2010.08.013] |
| [26] | Xia M, Guo V, Huang R, et al. Inhibition of morphine-induced cAMP overshoot: a cell-based assay model in a high-throughput format Cell Mol Neurobiol 2011; 31(6): 901-7. [http://dx.doi.org/10.1007/s10571-011-9689-y] |
| [27] | Xia M, Huang R, Kristine LW, et al. Compound cytotoxicity profiling using quantitative high-throughput screening Environ Health Perspect 2008; 116(3): 284-91. [http://dx.doi.org/10.1289/ehp.10727] |
| [28] | Sirbasku DA, Pakala R, Sato H, Eby JE. Thyroid hormone
dependent pituitary tumor cell growth in serum-free chemically
defined culture. A new regulatory role for apotransferrin Biochemistry 1991; 30(30): 7466-7. [http://dx.doi.org/10.1021/bi00244a015] |
| [29] | Xia M, Shahane SA, Huang R, et al. Identification of quaternary ammonium compounds as potent inhibitors of hERG potassium channels Toxicol Appl Pharmacol 2011; 252(3): 250-8. [http://dx.doi.org/10.1016/j.taap.2011.02.016] |
| [30] | Zhang JH, Chung TD, Oldenburg KR. A simple statistical parameter for use in evaluation and validation of high throughput screening assays J Biomol Screen 1999; 4(2): 67-73. [http://dx.doi.org/10.1177/108705719900400206] |
| [31] | Repa JJ, Turley SD, Lobaccaro JA, et al. Regulation of absorption and ABC1-mediated efflux of cholesterol by RXR heterodimers Science 2000; 289(5484): 1524-9. [http://dx.doi.org/10.1126/science.289.5484.1524] |
| [32] | Miller SC, Huang R, Sakamuru S, et al. Identification of known drugs that act as inhibitors of NF-kappaB signaling and their mechanism of action Biochem Pharmacol 2010; 79(9): 1272-80. [http://dx.doi.org/10.1016/j.bcp.2009.12.021] |
| [33] | McGlinchey PG, Webb ST, Campbell NP. 5-fluorouracil-induced cardiotoxicity mimicking myocardial infarction: a case report BMC Cardiovasc Disord 2001; 1: 3. [http://dx.doi.org/10.1186/1471-2261-1-3] |
| [34] | Bhat MK, Yu Cl, Yap N, et al. Tumor suppressor p53 is a negative regulator in thyroid hormone receptor signaling pathways J Biol Chem 1997; 272(46): 28989-93. [http://dx.doi.org/10.1074/jbc.272.46.28989] |
| [35] | Yap N, Yu CL, Cheng SY. Modulation of the transcriptional activity of thyroid hormone receptors by the tumor suppressor p53 Proc Natl Acad Sci USA 1996; 93(9): 4273-7. [http://dx.doi.org/10.1073/pnas.93.9.4273] |
| [36] | Blaner WS. Cellular metabolism and actions of 13-cis-retinoic acid J Am Acad Dermatol 2001; 45(5): S129-35. [http://dx.doi.org/10.1067/mjd.2001.113714] |
| [37] | Lee S, Privalsky ML. Heterodimers of retinoic acid receptors and thyroid hormone receptors display unique combinatorial regulatory properties Mol Endocrinol 2005; 19(4): 863-78. [http://dx.doi.org/10.1210/me.2004-0210] |
| [38] | Davis KD, Lazar MA. Selective antagonism of thyroid hormone action by retinoic acid J Biol Chem 1992; 267(5): 3185-9. |
| [39] | Davis KD, Berrodin TJ, Stelmach JE, Winkler JD, Lazar MA. Endogenous retinoid X receptors can function as hormone receptors in pituitary cells Mol Cell Biol 1994; 14(11): 7105-10. |
| [40] | Matsumoto S, Hashimoto K, Yamada M, Satoh T, Hirato J, Mori M. Liver X receptor-alpha regulates proopiomelanocortin (POMC) gene transcription in the pituitary Mol Endocrinol 2009; 23(1): 47-60. [http://dx.doi.org/10.1210/me.2007-0533] |
| [41] | Li D, Li T, Wang F, Tian H, Samuels HH. Functional evidence for retinoid X receptor (RXR) as a nonsilent partner in the thyroid hormone receptor/RXR heterodimer Mol Cell Biol 2002; 22(16): 5782-92. [http://dx.doi.org/10.1128/MCB.22.16.5782-5792.2002] |
| [42] | Castillo AI, Sánchez-Martínez R, Moreno L, et al. A permissive retinoid X receptor/thyroid hormone receptor heterodimer allows stimulation of prolactin gene transcription by thyroid hormone and 9-cis-retinoic acid Mol Cell Biol 2004; 24(2): 502-13. [http://dx.doi.org/10.1128/MCB.24.2.502-513.2004] |
| [43] | Koenig RJ, Brent GA, Warne RL, Larsen PR, Moore DD. Thyroid hormone receptor binds to a site in the rat growth hormone promoter required for induction by thyroid hormone Proc Natl Acad Sci USA 1987; 84(16): 5670-4. [http://dx.doi.org/10.1073/pnas.84.16.5670] |
| [44] | Furlow JD, Yang HY, Hsu M, et al. Induction of larval tissue resorption in Xenopus laevis tadpoles by the thyroid hormone receptor agonist GC-1 J Biol Chem 2004; 279(25): 26555-62. [http://dx.doi.org/10.1074/jbc.M402847200] |
| [45] | Lim W, Nguyen NH, Yang HY, Scanlan TS, Furlow JD. A thyroid hormone antagonist that inhibits thyroid hormone action in vivo J Biol Chem 2002; 277(38): 35664-70. [http://dx.doi.org/10.1074/jbc.M205608200] |
| [46] | Furlow JD, Neff ES. A developmental switch induced by thyroid hormone: Xenopus laevis metamorphosis Trends Endocrinol Metab 2006; 17(2): 40-7. [http://dx.doi.org/10.1016/j.tem.2006.01.007] |