- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

The Open Clinical Cancer Journal
(Discontinued)
ISSN: 1874-1894 ― Volume 5, 2011
Oral Mucositis, Pain and Xerostomia in Patients with Head and Neck Cancer who Received Chemoradiotherapy with or without Cetuximab
Ourania Nicolatou-Galitis*, 1, Triantafyllia Sarri1, Konstantinos Dardoufas2, Vassilis Kouloulias3, Xenophon Vakalis4, Argyro Polychronopoulou5, Dimitrios Demenagas6 , Anastasia Sotiropoulou-Lontou6
Abstract
Goal of work:
To compare the severity of oral mucositis, pain and xerostomia in head and neck cancer patients, who received radiotherapy with cisplatin and cetuximab to that of patients who received radiotherapy with cisplatin alone.
Patients:
Forty-nine head and neck cancer patients entered the study. Twenty-five patients (Group A) received radiotherapy and cisplatin. Twenty-four patients (Group B) received radiotherapy, cisplatin, and cetuximab.
Methods:
Oral mucositis was recorded weekly, according to EORTC/RTOG criteria. Pain and xerostomia were assessed using a 10cm visual analogue scale. Antifungal and antiviral treatment and prophylaxis were administered during RT to both groups.
Results:
During chemoradiotherapy, severe mucositis, pain and xerostomia were observed in 60%, 64% and 52% respectively in Group A, while the same symptoms were observed in 79%, 58% and 29% respectively in Group B. The differences between the two groups were not statistically significant. At the end of chemoradiotherapy, severe mucositis, pain and xerostomia were recorded in 24%, 32% and 32% in Group A and 37%, 21% and 17% respectively in Group B. The differences between the two groups were, again, statistically not significant. Neither significant differences were found between the two groups with respect to the use of antifungal and antiviral treatment, radiotherapy interruptions and weight loss. In both groups, oral mucositis, pain and xerostomia were significantly reduced at the end of radiotherapy as compared to those during RT, following the anti-infectious treatment and prophylaxis.
Conclusion:
Cetuximab, added to cisplatin/radiotherapy, did not increase the severity of oral mucositis, pain and xerostomia, in head and neck cancer patients, with limitations of the study design and its limited number of patients.
Article Information
Identifiers and Pagination:
Year: 2010Volume: 4
First Page: 6
Last Page: 14
Publisher Id: TOCCJ-4-6
DOI: 10.2174/1874189401004010006
Article History:
Received Date: 2/8/2010Revision Received Date: 18/2/2010
Acceptance Date: 29/3/2010
Electronic publication date: 26/8/2010
Collection year: 2010
open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution Non-Commercial License(http://creativecommons.org/licenses/by-nc/3.0/), which permits unrestrictive use, distribution, and reproduction in any medium, provided the original work is properly cited.
* Address correspondence to this author at the School of Dentistry, University of Athens, Bouboulinas 41, N. Psyhico, 154 51 Athens, Greece; Tel: +30 210 6748715; Fax: +30 210 6775567; E-mail: nicolatou.galitis@lycos.com
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 2-8-2010 |
Original Manuscript | Oral Mucositis, Pain and Xerostomia in Patients with Head and Neck Cancer who Received Chemoradiotherapy with or without Cetuximab | |
INTRODUCTION
Chemotherapy (Chemo) has become an integral part of potentially curative therapy for head and neck cancer. Che-moradiotherapy (ChemoRT) has been shown to be superior over radiotherapy (RT) alone, in terms of locoregional control and overall survival. Platinum based regimens in combination with 5-fluorouracil have been the most effective chemotherapy regimen so far. Given the toxicity of con-current ChemoRT, careful selection of patients is critical [1-3].
Targeted therapies, which include monoclonal antibodies and small molecule inhibitors, that target specific growth factors and growth factor receptors, have significantly changed the treatment of cancer over the past 10 years. One particular growth factor receptor and signal transduction system that has been shown to play an important role in the pathogenesis of head and neck cancer is the epidermal growth factor receptor (EGFR) and its ligand. Ligand binding to EGFR results in activation of tyrosine kinase pathways that significantly affect tumorigenesis by stimula-ting cell proliferation, inhibiting apoptosis and favoring angiogenesis to facilitate metastasis.
EGFR is overexpressed (80-100%) and/or abnormally activated in head and neck cancers, correlating with poor clinical outcome in these patients [4-6]. Inhibition of the EGFR-induced signal transduction pathways by monoclonal antibodies has been shown to inhibit the growth of EGFR-expressing human cancer cells.
Cetuximab (C-225, Erbitux®), is an IgG1 subclass mouse-human chimeric monoclonal antibody, which binds with high affinity to the extracellular ligand binding domain of the receptor, leading to inhibition of cancer cell prolife-ration, invasion and migration.
Cetuximab competes with natural legands of EGFR for binding to the receptor and thus, prevents activation of the receptor. In addition, cetuximab might trigger the internaliza-tion and degradation of the receptor. An antineoplastic effect mediated by immune mechanisms has also been postulated, specifically by the induction of antibody-dependent cell-mediated cytotoxicity [7].
On the other hand, EGFR is also expressed in normal epithelial cells (of skin and mucosa), thus, EGFR inhibition can lead to significant dermatologic and gastrointestinal toxicities.
Several studies and clinical trials have been conducted to investigate the effect and safety of cetuximab used for the treatment of locoregionally advanced and/or recurrent or metastatic squamous cell carcinoma of the head and neck or nasopharyngeal carcinoma, in terms of locoregional control, free-disease survival, and overall survival [8-7].
Cetuximab has been used alone and in combination with platinum-based chemotherapy [9-1, 13] or in combination with platinum-based chemotherapy and 5-fluorouracil (5-FU) [12, 14, 17]. Cetuximab has also been used in combina-tion with radiotherapy [8, 15, 16].
Cetuximab has been found to be well tolerated and active. A superior overall survival, progression-free survival and locoregional control, attributed to cetuximab, have been reported [14-17].
When cetuximab was added to platinum-based chemo-therapy and 5-FU, there was no significant difference in the overall incidence of grade 3 or 4 adverse events between the groups [14]. The addition of cetuximab had no impact on the Quality of Life, as it was assessed with the QLQ-C30 and QLQ-H&N35 questionnaires. Furthermore, patients in the cetuximab arm displayed significant improvements in pain, swallowing problems and scores for speech and social eating problems [17].
Skin reactions, particularly an acne-like rash, as a result of the normal expression of EGFR in the skin, are the most common cetuximab-related adverse events, in cetuximad and chemotherapy combinations, reported in more than half of the patients, mainly grade 1 and 2 [9-4, 17].
The combination of cetuximab and radiotherapy in patients with locoregionally advanced head and neck car-cinoma, did not increase the common toxic effects associated with RT, including oral mucositis, xerostomia, dysphagia, oral pain/odynophagia, weight loss and performance status deterioration [8, 15, 16].
The addition of cetuximab did not adversely affect the quality of life. This was particularly notable for global health status/QoL, social functioning, social eating and social contact [16]. Acneiform rash was found to cause a dose reduction in less than 5% of the patients.
In contrast to the previously documented efficacy and safety of cetuximab, Pryor et al. [18] reported that the addition of cetuximab to radiotherapy, administered to 13 patients, with locally advanced head and neck carcinoma and major co-morbidities, including dysphagia and significant weight loss prior to the commencement of therapy, resulted in a high rate of toxicity, associated with low treatment compliance and delays in completing RT. Grade 3 oral mucositis developed in 10 patients (77%) frequently in areas that had received less than 10-15Gy, most commonly the mucocutaneous junction of the lips.
The mucocutaneous junction of the lips is, however, the site of predilection of herpes simplex virus-1 (HSV-1) reactivation and infection (Herpes Labialis). Herpes simplex virus-1 reactivation and infection is frequent in cancer patients [19] and can, often, complicate the ulcers of oral mucositis, increasing the severity of mucositis [20, 21]. Accordingly, HSV-1 infection could be related to the severe mucositis on the mucocutaneous junction of the lips in those 10 patients reported by Pryor et al. [18].
Significant adverse effects, including one myocardial infarction, one bacteremia, one atrial fibrillation, and two deaths (one of pneumonia) were also reported when cetuximab was administered concurrently with radiotherapy and platinum-based chemotherapy in 22 patients, with predominantly stage IV squamous cell carcinomas of the head and neck, although preliminary efficacy was encoura-ging [22]. The study was closed because of significant adverse events. The potential microbial systemic dissemina-tion from the oral cavity in patients with advanced cancers and co-morbidities was not considered. As it is known, the oral mucosal barrier damage and the development of oral ulcerative mucositis may allow for the development of local and / or systemic fungal or viral or bacterial infections.
Severe oral ulcerative mucositis, grade 3 or 4, is a major limitation to continuous, uninterrupted RT and concurrent chemotherapy in the management of head and neck cancer. Nearly all patients (90-97%) with head and neck cancers receiving RT or chemoRT will experience some degree of mucositis [23-25]. Over 50% of these patients may expe-rience severe, grade 3-4 mucositis, associated severe pain, dysphagia and severe weight loss, which compromises patient’s quality of life. Higher grades of ulcerative mucositis are also related to higher frequency of radiation treatment breaks with adverse effect on tumor control [26, 27].
In addition, mucositis has a major economic impact due to costs associated with pain management, liquid diet supp-lements, gastrostomy tube placements or total parenteral nutrition, management of secondary infections and hospita-lizations [28, 29].
Local infections, candidiasis and herpes simplex may complicate the clinical course of oral mucositis, while oral flora colonizing the mucosa may lead to secondary poten-tially life threatening systemic infection [30-32], especially in compromised patients, with advanced or metastatic cancer.
The most common and well studied infection of the oral mucosa during head and neck cancer RT is oral candidiasis, reported with an incidence of 27%-53% [20, 33-36].
Oral candidiasis develops superimposed on mucostis, in a Candida carrier, potentially adding to the severity of mucositis. Candida carriage prevalence, in head and neck RT, varies between 50% to 70% [36-38]. Systemic adminis-tration of fluconazole prophylaxis has prevented candidiasis, and has significantly reduced the Candida carriage and the severity of oral mucositis and RT interruptions [36, 39, 40].
Herpes simplex virus-1 (HSV-1) reactivation, both before and after cancer therapy, has been reported to be frequent. Patients, who received cancer therapy and were found HSV positive with direct immunofluorescence, seemed to have more severe oral mucositis than HSV negative patients, while a positive IgM result was more frequent in the mucositis group of patients [19].
Herpes simplex virus-1 infection in radiation-induced oral mucositis, complicating the severity of mucositis has been shown in our head and neck cancer patient population [20, 21]with an incidence of 29%. HSV-1 infection has been observed to aggravate mucositis.
Based on the presented data of the high incidence of candidiasis, and Candida carriage, the risk of infection recurrences, as well as the high risk of HSV-1 reactivation and infection and the infections’ potential role in the severity of oral mucositis, combined with the difficulty in the differential diagnosis of mucositis from infections [21, 36], systemic antifungal and antiviral treatment and prophylaxis has been introduced to our head and neck cancer patient population as a routine clinical practice, since 2005.
The purpose of this report was the assessment of oral toxicity in head and neck cancer patients who received cetuximab, added to radiotherapy and platinum-based che-motherapy. The oral toxicity was prospectively evaluated in 24 head and neck patients and was compared to that of 25 patients, who received RT with platinum-based chemother-apy alone, in the years 2007 and 2008. The patients, in both arms, received antifungal and antiviral treatment and prophylaxis.
PATIENTS AND METHODS
Patients and Eligibility Criteria
Forty-nine consecutive patients with malignant head and neck tumor, eligible to receive chemoradiotherapy were included in the study. The patients were reffered to the Dental Oncology Unit for standard oral oncology supportive care before and during the course of chemoradiotherapy from four Athens Cancer Centers between January 2007 to September 2008.
General blood tests, liver and renal functions were within normal limits. Karnofsky perfomance status ranged between 80-100%.
Twenty-five patients (Group A) received RT and concomitant chemotherapy, which consisted of cisplatin 75mg/m2 every three weeks.
Twenty-four patients (Group B) received RT and con-comitant chemotherapy, which consisted of cisplatin 75mg/m2 every three weeks and cetuximab at an initial dose of 400mg/m2, followed by 250 mg/m2 every two weeks.
The decision whether the patient would receive RT and cisplatin or RT, cisplatin and cetuximab relied upon each patient’s radiation oncologist.
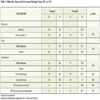
Patient data are summarized in Table 1.
The median age was 63 years (range 42-83 years) for Group A (RT plus cisplatin) and 57 years (24-78 years) for Group B (RT plus ciplatin and cetuximab). The majorities of the patients were male in both groups (68% and 71%, respectively) and had squamous cell carcinoma according to histological diagnosis (56% and 46%, respectively). In addition, the majority of our patients were in T1 and T2 of their cancer (76% of Group A and 62.5% of Group B).
The two groups did not differ significantly in respect to most of the baseline characteristics of the patients. Signifi-cantly more patients from Group B were, however, free of nodal disease.
All patients provided written informed consent.
ChemoRadiotherapy
Patients were irradiated with a 6-MV linear accelerator. Twenty-five patients received radical and 24 patients received postoperative radiotherapy. The primary tumor and draining lymphatics were treated with parallel-opposed fields. Supraclavicular and low-neck nodes were treated with an anterior field.
The daily and the total radiation dose are shown in Table 1.
In Group A the majority of the patients (68%) received a daily dose of 1.8 Gy. The mean total dose was 65.6 Gy (range 59,4 to 72 Gy).
In Group B the majority of the patients (83%) received a daily dose of 2.0 Gy. The mean total dose was 64.6 Gy (range 46 to 70 Gy). Significantly more patients from Group B were irradiated with a daily dose of 2.0Gy.
The lateral field doses were reduced after 40-43 Gy to avoid overdose to the spinal cord. The regional nodes were irradiated to a total dose of 45-61 Gy, depending on the nodal stage.
Group A (RT and cisplatin) received concomitant cispla-tin at a dose of 75mg/ m2 every three weeks.
Group B (RT plus cisplatin and cetuximab) received concomitant cisplatin at a dose of 75mg/ m2 every three weeks and cetuximab at an initial dose of 400mg/m2, followed by 250mg/m2 every two weeks.
Oral Clinical Evaluation
Data on oral toxicity were prospectively collected, outside the context of a clinical trial. Patients were examined weekly.
Oral mucosal evaluation was performed by the oral medicine specialist and mucosal evaluation included:
The scoring of oral mucositis, according to EORTC/ RTOG criteria as follows: Grade 1 (diffuse erythema, patient can eat solid food), Grade 2 (erythema and small foci or ulcers, patient can take soft diet), Grade 3 (painful ulcers extending on more than half of the oral mucosa, patient can take liquids only), Grade 4 (painful ulcers covering almost all mucosal surfaces, alimenta-tion is not possible).
The presumptive diagnosis of oral pseudomembranous candidiasis, which was made, as described before [20, 35, 36], when easily removable, mostly painless, whitish pseudomembranes were observed.
The presumptive diagnosis of HSV-1 infection, which was evaluated according to the clinical criteria previously reported [20, 21]. Those criteria included: (i) abrupt appearance of severe, extensive ulcers and/or (ii) early initiation of ulceration, within the first 2 weeks of RT, ulcers on the dorsum of the tongue, or on the hard palate, or on the vermillion border.
According to the policy of our Clinic, antifungal and antiviral treatment and prophylaxis, based on clinical criteria, as described, were administered. Antifungal and/or antiviral treatment was administered according to the drug’s ins-tructions.
Antifungal treatment consisted of itraconazole 200mg/ day for one week or posaconazole 200mg the first day and 100mg/day for another 13 days.
Antiviral treatment consisted of acyclovir 2gr per day for one week.
Antifungal and antiviral medication continued as prophylaxis, reduced to the half dose, until the end of RT.
Standard oral mucosal and dental care, including blant mouthrinses and topical fluorides, were introduced to all patients.
Pain and xerostomia were self-assessed using a 10cm visual analogue scale by the patients. A score from 0 to 4 was evaluated as mild pain or xerostomia, a score from 5 to 7 was evaluated as moderate pain or xerostomia and a score from 8 to 10 was evaluated as severe pain and xerostomia.
Statistical Analysis
hi-squared test was employed to compare the two groups of interest, with respect to baseline characteristics, incidence of severe mucositis, pain and xerostomia during and after the RT, the use of antifungals and antivirals, the incidence of interruptions overall and the incidence of interruptions due to severe mucositis, and the weight loss. Total dose and age were assessed by t-test while daily dose, tumor and node stage were assessed by Fisher’s exact test.
The prevalence of severe mucositis, severe pain and severe xerostomia at the end of RT was assessed by McNemar’s chi-squared test.
All statistical tests were two-sided, and the level of statistical significance was set at 5%.
RESULTS
ChemoRadiotherapy
Group A (RT and cisplatin)
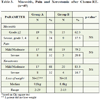
Twenty-one of 25 patients (84%) completed chemoradio-therapy within the preplanned time. Four patients (16%) interrupted RT for one week; three of them had a treatment gap due to severe mucositis (Table 2).
Group B (RT, cisplatin and cetuximab)
Nineteen of 24 patients (79,2%) completed chemoradio-therapy within the preplanned time. Five patients (20.8%) interrupted their therapy for one week; four of them had a treatment gap because of severe mucositis (Table 2).
The difference of RT interruption due to severe mucositis between the two groups was not found significant.
Skin Reactions in Group B
Fifteen of 24 patients developed severe skin toxicity, including dry desquamation, early moist desquamation, blister formation, skin pealing and bleeding ulcers. Patients were encouraged to maintain good standards of hygiene following the consensus guidelines [41]. They were treated with topical application of fucidic acid and betamethazone (Fucicort Cream) and/or betamethazone valerate (Betnovate cream), twice a day. Skin toxicity was well tolerated and no one patient discontinued therapy because of severe skin reactions.
Oral Toxicity During the Course of RT (Table 2)
Oral Mucositis
Group A:
Twenty-two of 25 patients (88%) developed ulcerative mucositis (grade 2, 3 or 4).
Mild to moderate mucositis was evaluated in 10 patients (40%), while severe mucositis grade 3 or 4 was scored in 15 patients (60%), as it is shown in Table 2.
Group B:
Twenty-three of 24 patients (96%) developed grade 2, 3 or 4 mucositis.
Mild to moderate mucositis was evaluated in 5 patients (20.8%), while severe mucositis grade 3 or 4 was scored in 19 patients (79.2%), as it is shown in Table 2.
The incidence of severe mucositis during RT between the two groups did not differ statistically.
Oral Pain
Mild and moderate pain was reported by 9 of 25 patients (36%) in Group A and by 10 of 24 patients (41.7%) in Group B.
Severe pain was reported by 16 of 25 patients (64%) in Group A and by 14 of 24 patients (58.3%) in Group B.
The incidence of severe pain between the two groups during RT was not statistically significant.
Xerostomia
Mild and moderate xerostomia was reported by 12 out of 25 patients (48%) in Group A and by 17 out of 24 patients (70%) in Group B.
Severe xerostomia was reported by 13 of 25 patients (52%) in Group A and by 7 of 24 patients (30%) in Group B. The incidence of severe xerostomia between the two groups during RT was not statistically significant.
Antifungals
Systemic antifungals were used in 20 patients (80%) of Group A and in another 20 patients (83%) of Group B.
Antivirals
Systemic antivirals were administered to 20 patients (80%) of Group A and to 18 patients (75%) of Group B.
The differences in antifungal and antiviral use between the two Groups were not significant.
Oral Mucositis
Group A
Overall ulcerative mucositis grade 2, 3 and 4 was evaluated in 19 patients (76%).
Mild to moderate mucositis was evaluated in 19 patients (76%), while the incidence of severe mucositis grade 3 or 4 was significantly reduced to 24%, observed in 6 patients, as it is shown in Table 3and 4 (p=0.003).
Group B
Overall ulcerative mucositis grade 2, 3 and 4 was evaluated in 19 patients (79.2%).
Mild to moderate mucositis was evaluated in 15 patients (62.5%), while the incidence of severe mucositis grade 3 or 4 was significantly reduced to 37.5%, observed in 9 patients, as it is shown in Tables 3 and T4 (p=0.002).
The differences between the two groups in terms of the incidence of severe mucositis at the end of RT were not statistically significant.
The reduction of the severity of mucositis, attributed to the antifungal and antiviral prophylaxis, at the end of RT, was significant in both groups A and B.
Oral Pain
Mild and moderate pain was reported by 17 patients (68%) in Group A and by 19 patients (79.2%) in Group B.
The prevalence of severe pain was significantly reduced to 32% in Group A and to 20.8% in Group B, p=0.003.
The differences between the two groups in terms of the prevalence of severe pain at the end of RT were not statistically significant.
Xerostomia
Mild and moderate xerostomia was reported by 17 patients (68%) in Group A and by 20 patients (83.3%) in Group B.
In Group A severe xerostomia was significantly reduced to 32%, p=0.03. In Group B xerostomia was also reduced, though not significantly, to 16.7%.
The differences between the two groups in terms of the prevalence of severe xerostomia at the end of RT were not statistically significant.
Weight Changes
They were available in 18 patients from each group (Table 3).
Group A: Loss of weight was observed in 17 patients and ranged between 2 to 29kg, with a mean loss of 9,8kg. One patient did not lose weight.
Group B: Loss of weight was observed in 18 patients and ranged between 2 to 15kg, with a mean loss of 8,6kg.
The weight loss did not differ significantly between the two groups.
DISCUSSION
The present observations, which were collected pros-pectively, in a consecutive patient cohort, “out in the real world”, during the routine chemoradiotherapy schedule, outside the context of a clinical trial, showed that the addition of cetuximab in the chemoradiotherapy scheme did not increase the oral toxicity.
The incidence of severe oral mucositis, objectively observed, by Oral Medicine specialist, and severe pain and xerostomia, self-reported by the patients, in group B, who received RT, cisplatin and cetuximab, did not differ significantly, when compared to those of Group A, who received RT and cisplatin, both during and after the completion of chemoRT.
The RT unplanned breaks and the RT interruptions due to severe mucositis did not differ significantly between the two patient groups, either.
Similar numbers of patients, in both groups, received antifungals and antivirals. The use of anti-infectious medi-cations, being similar in the two groups, further indicated, though indirectly, that cetuximab did not increase the local, mucosal toxicity, since oral mucosal infections consist a significant part of oral toxicity in cancer patients [19-21, 33-38, 42].
The prevalence of severe oral mucositis, pain and xerostomia observed during the course of chemoRT were significantly reduced in both groups after the completion of chemoRT, attributed to the administration of antifungal and antiviral treatment and prophylaxis, as has peviously been shown [20, 21, 36, 39, 40].
The patients, in the two groups, did not differ signi-ficantly in most characteristics, such as the gender, age, tumor histological diagnosis and tumor size.
The numerical difference in the median age, although not significant, represents a weakness, which is related to the non-randomized controlled study design. It is not known how or whether the younger age of the patients in Group B as opposed to Group A (57 vs. 63 years) could have affected the oral toxicity and the tolerance of the patients to the combination of cetuximab, cisplatin and RT.
The significantly higher number of patients in group B (cetuximab, cisplatin and RT) without nodal involvement as opposed to Group A (8 vs. 2 patients) represents another weakness of the non randomized study design. On the other hand, significantly more patients from Group B were irradiated with a 2.0Gy daily RT dose (20 vs. 8 patients). The limited number of patients in both groups did not, however, allow for further comments and evaluations.
Cetuximab was well tolerated in our patients, as in previous reports, where cetuximab was added to RT [8, 15, 16] or to platinum-based chemotherapy [9-1, 13] or to platinum-based chemotherapy and 5-FU [12, 14, 17] and it did not increase the common toxic effects. Significant adverse effects, as they had been previously reported when cetuximab was administered concurrently with radiotherapy and platinum-based chemotherapy [18, 22], were not observed in the present study.
Interestingly, a great majority of patients in both groups (76% from Group A and 62.5% from Group B) were in the advantageous tumor size T1 or T2 of their cancer. Further-more, all patients were not previously treated and had no serious co-morbidities. This is in contrast to the patients of previous reports, who had locoregionally advanced head and neck cancer or previously treated recurrent and/or metastatic cancer, with potentially compromised nutrition and well being [8-7, 18, 22]. Although, as is generally accepted [1], careful selection of patients is critical in chemoRT, the extent to which the early stage of the cancer patients in the present report might have influenced toxicity, cannot be evaluated in this setting. It would be interesting to assess, in a next step, given the increasing use of cetuximab, whether patients with different stages in their cancers exhibit different tolerance values and toxicities after cetuximab administration.
Seventy per cent of the patients of both groups, in the present study, received antifungals and antivirals during the course of chemoRT, according to the policy of our Center. The need and the use of antifungals and antivirals have not been mentioned in the previous reports [8-7, 18, 22].
Local, oral mucosal infections and their potential sys-temic dissemination, especially in patients, compromised with advanced cancers, previous treatments and serious co-morbidities, were not clearly ruled out in previous studies.
The severe adverse effects reported by Pfister et al. [22], could be related, at least in two of the cases (bacteremia and pneumonia), to systemic infection disseminated from a possible oral site-mucosal infection, superimposed on oral mucositis, while HSV-1 infection could be the cause of severe labial toxicity reported by Pryor et al. [18]. In addition, 3 of the 13 patients reported by Pryor et al. [18] had dysphagia and severe weight loss before the commen-cement of treatment.
The high incidence of rash and dermatitis of our patients (62.5% of the Group B) is in agreement with the recent report by Giro et al. [43]. The acne-like rash and dermatitis, observed in our patients was adequately managed, according to the suggested guidelines [41]. Severe skin reactions as reported in the cases by Berger et al. [44]and by Budach et al. [45] were not observed.
In conclusion, no statistical differences for the toxicities under study were observed. Cetuximab did not increase the severity of the oral mucositis, pain and xerostomia in head and neck cancer patients, who received chemoradiotherapy, with limitations of the current study design and its limited number of patients. A large-scale randomized controlled trial is needed to confirm the above results.
Risk factors, including co-morbidities and the extent of locally advanced head and neck cancer stage, potentially compromising the patient, and other factors should be studied, identified and taken into account, in order to use cetuximab at an optimal setting for the optimal patient benefit.