- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

The Open Process Chemistry Journal
(Discontinued)
ISSN: 1875-1806 ― Volume 6, 2014
Modeling of Amino Acid Separation Process in Single and Dual Chiral Ligand Exchange Chromatographic Systems
Pepa Dimitrova, Hans-Jörg Bart*
Abstract
In the present paper the separation of three amino acids applying ligand exchange chromatography in single and dual chiral systems was modeled using the dispersive equilibrium model. The investigated systems consist of a commercially available non chiral column and a commercially available chiral column using a chiral mobile phase. With the experimentally determined model parameters and the multi-component competitive Langmuir adsorption isotherm a very good mathematical prediction of the break-through curves and analytical injections at low concentrations was achieved. At higher concentrations the model application is limited due to displacement effects and system complexity.
Article Information
Identifiers and Pagination:
Year: 2011Volume: 4
First Page: 1
Last Page: 7
Publisher Id: TOCPCJ-4-1
DOI: 10.2174/1875180601004010001
Article History:
Received Date: 30/5/2011Revision Received Date: 30/7/2011
Acceptance Date: 11/8/2011
Electronic publication date: 30/8/2011
Collection year: 2011
open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution Non-Commercial License (http: //creativecommons.org/licenses/by-nc/3.0/ which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
* Address correspondence to this author at the TU Kaiserslautern, Thermische Verfahrenstechnik, P.O. Box 3049; 67653 Kaiserslautern, Germany; Tel: +49 6312052414; Fax: +49 6312052119; E-mail: bart@mv.uni-kl.de
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 30-5-2011 |
Original Manuscript | Modeling of Amino Acid Separation Process in Single and Dual Chiral Ligand Exchange Chromatographic Systems | |
1. INTRODUCTION
High performance liquid chromatography (HPLC) offering high efficiency is the mostly applicable chromatographic technique in different industries dealing with optical pure substances, as are enantiomers, although its effectiveness is linked to expensive stationary phases. This is the reason why alternative methods are under research and development revealing other possibilities. Such are solvent impregnated resins (SIR), which were found to be effective not only for separation of base metals and organic solutes but also for amino acids [1-3]; micellar enhanced ultrafiltration [4, 5]; microemulsion electrokinetic chromatography [6]; extraction with micelles and microemulsions [7-10]; emulsion liquid membranes [11] and micellar chromatography [12]. However, at that point, it worth mentioning, that up to now, HPLC remains the main technique for enantiomeric separations.
The separation of enantiomers from racemic mixtures is quite sophisticated as these substances have identical physical and chemical properties. In order to implement the separation a chiral selector that can distinguish them has to be used. For instance, in the SIR technique this selector can be physically impregnated on inert solid supports resulting in a new composite material. In the microemulsion the selector can be immobilized into the droplets and in the micellar chromatography into the micellar core.
A possibility for optimization (faster separation and less mobile phase resulting in reduced process costs) of a separation process could provide the combination of two chiral selectors in one chromatographic separation system. In the present paper the enantiomeric separation of amino acid racemates is investigated applying single and dual chiral ligand exchange chromatography (LEC) systems. To achieve a LEC process in the single system a chiral mobile phase and non-chiral column were used and in the second a chiral stationary phase and a chiral mobile phase [13]. Both chiral selectors form different stable complexes with the L- and D-amino acid enantiomers in the presence of copper. The chiral mobile phase additive (N, N-dimethyl-L-phenylalanine) builds more stable complexes with the D-forms and the covalently bound selector on the chiral column (Astec CLC-L) has an opposite selectivity with the L-form. Concentration loading experiments have shown that only the dual system can be used for preparative separations, whereas the single one is appropriate for fast analytics [13].
For the design and optimization of such chromatographic processes the knowledge of equilibrium data, i.e. the adsorption isotherms, is of major importance [14]. In a previous work these were experimentally determined for three amino acids (D,L-leucine, D,L-methionine and D,L-tyrosine) applying the dynamic methods frontal analysis and perturbation [13]. In the present paper the determined equilibrium data were modeled with the appropriate isotherm equation and the adsorption parameters were used for the application of the equilibrium dispersive model for the mathematical simulation of the separation process.
In different reviews devoted to theoretical problems of nonlinear chromatography it is elucidated, that a simulation of a chromatographic separation process requires mainly the knowledge of the thermodynamic equilibria between the mobile phase, the stationary phase and the components of the mixture to be separated, e.g. the adsorption isotherms [14].
1.1. Isotherm Model
As in a racemic mixture there is a competition between both enantiomers for the adsorption sites, which has to be considered, it is usually the most important step to include correctly the binary interaction into a model. That is why in the current paper the multi-component competitive Langmuir equation (eq. 1) which takes into account the influence of the adsorption of one component on the adsorption behaviour of the others was used:
Where bi is the equilibrium parameter, which is the ratio between the velocity constants of adsorption and desorption and qs,i is the saturation capacity for each component. In a racemic mixture one deals with two components, therefore four equilibrium parameters have to be determined – HD, HL, bD and bL. HD, and HL were calculated from the retention data given in a previous work [13] using eq. (2):
Where to is the retention time of a non-adsorbable component, tRi is the enantiomer retention time (i=D,L) and ɛtis the total column porosity.
Assuming equal saturation capacities for each enantiomer of one amino acid both equilibrium parameter bD and bL were determined by nonlinear regression using the solver in Microsoft Excel 2007 to minimize the sum of the relative errors by varying the model parameters.
1.2. Equilibrium Dispersive Model
The band profiles obtained in chromatography and in other adsorption separation processes can be calculated by different mathematical models [15]. The most important of them are the equilibrium dispersive model (ED), the general rate model (GR), the inverse method (IM) and the transport-dispersive model (TD) [16-18]. The choice between these models is critical. The most rigorous model will not necessarily give the best agreement between calculated profiles and experimental ones. More rigorous models take more phenomena into account but they require also more parameters, which are often difficult to obtain. Often, these parameters are estimated using correlations and the limited accuracy and precision obtained is not in accordance with the computational costs [19]. At high concentrations the influence of the thermodynamics of phase equilibrium on the band profile, i.e. the influence of the non-linear behaviour of the isotherms is decisive [20].
The ED as the simplest realistic model is the most often used one. Due to the high efficiency of the HPLC columns this model always gives good results, especially for the separation of small molecules. Furthermore, it is often in good agreement with the experimental data when mass transfer is mainly controlled by diffusion in the mobile phase and the mass transfer resistances are small, which is often the case in separating low molecular weight components like amino acids [15]. That is why this model was used in the current work.
The ED assumes instantaneous equilibrium between stationary and mobile phases, and the effects of all the factors which contribute to axial mixing are included together in an apparent dispersion coefficient Dai(eq.3) and the two phases are assumed to be constantly in equilibrium [20]. The time dependent change of the concentration in the liquid, ci(t,z), is given with convection, adsorption onto the solid phase, qi(t,z), and dispersion by the equation:
where t and z are the time and the length coordinates, Dai is the apparent dispersion coefficient for the component i (i=D,L) and can be calculated from the number of theoretical plates N (Dai=uL/2N), F is the phase ratio (F=1-ɛt/ɛt) and u is the interstitial velocity. In the case of a non-linear isotherm for a multi-component mixture of the K species eq. (2) can only be solved numerically using the appropriate initial and boundary conditions [21]. The model was solved using back-differences for spatial discretisation and solving the resulting system of ordinary differential equations in MATLAB using the ode15s-solver [21, 22].2. MATERIALS AND METHODOLOGY
2.1. Equipment
A high performance liquid chromatographic unit equipped with an UV/VIS photodiode array detector SPD-M10Avp (Shimadzu), solvent delivery module LC-10ADvp (Shimadzu), an on-line vacuum degasser DGU-20A3 Prominence (Shimadzu) and an autosampler SIL-20A Prominence (Shimadzu) was used for the experiments. An additional injection valve allowing very low extra volumes was connected enabling accurate isotherms determinations. The chromatographic equipment and its operation were already discussed in a previous work [13]. All experiments were performed at room temperature (25oC).
2.2. Materials
The amino acids tested D,L-leucine (D,L-Leu), D,L-methionine (D,L-Met) and D,L-tyrosine (D,L-Tyr) were supplied by Fluka. Copper (II) acetate, acetic acid and N,N-dimethyl-L-phenylalanine were purchased by Sigma Aldrich Chemie All chemicals were of analytical grade. Bi-distilled water was used for the preparation of solutions and washing of the experimental equipment.
2.3. Columns
Two columns were used for the determination of the adsorption isotherms – one equipped with a non-chiral reverse phase (Zorbax-Eclipse XDB-C8, 150 x 4.6 mm, 5 μm) from Agilent Technologies and the other with a chiral reverse phase (Astec CLC-L, 150 x 4.6 mm, 5 μm) from Sigma Aldrich. Their parameters are given elsewhere [13].
2.4. Mobile Phase Preparation
An aqueous chiral mobile phase containing a copper complex of the chiral additive N,N-dimethyl-L-phenylalanine with both columns was used. For the experiments with the non-chiral column the copper (II) concentration was 0.1 mmol L-1 and for the experiments with the chiral column 1 mmol L-1, whereas the N,N-dimethyl-L-phenylalanine concentration was always 2 mmol L-1. The flow rate was for the single LEC system 1 mL min-1 and for the dual one 2 mL min -1.
2.5. Measurements of Isotherm Data
Complete details regarding the experimental work can be found in a previous publication [13]. The adsorption isotherms for the single and dual LEC systems were determined applying the dynamic methods frontal analysis (FA) and the perturbation method (PE). For the dual system measurements only in the linear range were possible due to displacement effects [13].
3. RESULTS AND DISCUSSION
3.1. Adsorption Isotherms Modeling
The competitive equilibrium isotherm data obtained from the competitive frontal analysis measurements are treated with the multi-component Langmuir model. The determined parameters are given in Table 1. The relative error (RMSD) between the simulated and the experimental values is also given. In 1 the values of the equilibrium constant for each component differ quite significantly for both systems. It demonstrates that the enantioselectivity is controlled by the difference between bD and bL.
An analysis shows that most isotherms have a Langmuir behaviour. Interestingly in the single chiral LEC system all D-enantiomers have anti-Langmuir and all L-enantiomers Langmuir behaviour. In the literature such effect for Met was already noticed [21]. For the dual chiral system only D-Tyr and L-Met had an anti-Langmuir behaviour.
3.2. Validation of the Model
The parameters of isotherm determined by competitive frontal analysis are applied to ED model with the competitive Langmuir isotherm. The apparent dispersion coefficients from all amino acids are summarized in Table 2. The ED is validated by comparing the calculated data with the experimental ones for both chiral systems as well as for the break-though curves and for analytical injections.
3.3. Single Chiral LEC System
Fig. (1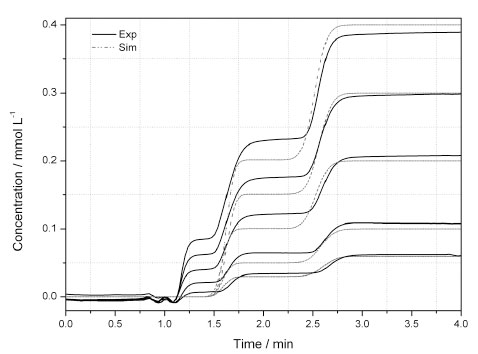 ) shows a comparison between the calculated (dash line) and experimental data (solid line) for the amino acid D,L-Leu at different feed concentrations. A good agreement is shown only for the second plateau with the L-enantiomer. Obviously due to more complex interaction between the D-enantiomer and the selector a deformation in the first plateau is observed which cannot be described by the ED model. However, the slopes and the retention times are well predicted for the L-plateau. One can see that with increasing the feed concentration the deviation between calculated and experimental data profiles also increased, which is due to calibration errors or base line drift. Furthermore, when determining the van Deemter plot it was shown in a previous publication that the mass transfer resistance has a bigger influence on the apparent dispersion coefficient than the axial dispersion [23], which could also contribute to the deviation between experimental and simulated D- plateau.
) shows a comparison between the calculated (dash line) and experimental data (solid line) for the amino acid D,L-Leu at different feed concentrations. A good agreement is shown only for the second plateau with the L-enantiomer. Obviously due to more complex interaction between the D-enantiomer and the selector a deformation in the first plateau is observed which cannot be described by the ED model. However, the slopes and the retention times are well predicted for the L-plateau. One can see that with increasing the feed concentration the deviation between calculated and experimental data profiles also increased, which is due to calibration errors or base line drift. Furthermore, when determining the van Deemter plot it was shown in a previous publication that the mass transfer resistance has a bigger influence on the apparent dispersion coefficient than the axial dispersion [23], which could also contribute to the deviation between experimental and simulated D- plateau.
 |
Fig. (1) Comparison between experimental (solid lines) and simulated (dash lines) breack-through concentraion profiles for D,L-Leu. |
When applying the ED model for prediction of the analytical band profiles a good agreement between experimental and calculated data was observed only for the L-enantiomer (see Fig. (2 )). The retention times and the shapes of the peaks were well predicted, but a deviation in the area of the D-enantiomer peak was observed. The model predicts a higher amount adsorbed on the stationary phase. The results show that the diastereomeric complexes with the D-forms are preferably formed in the mobile phase.
)). The retention times and the shapes of the peaks were well predicted, but a deviation in the area of the D-enantiomer peak was observed. The model predicts a higher amount adsorbed on the stationary phase. The results show that the diastereomeric complexes with the D-forms are preferably formed in the mobile phase.
 |
Fig. (2) Comparison between experimental (solid lines) and simulated (dash lines) concentraion profiles for D,L-Leu (3.8 mmol L-1). |
In Fig. (3 ), the experimental and the simulated break-through profiles for the D,L-Met are presented. Here again the same effect for the first plateau with the D-enantiomer can be seen.
), the experimental and the simulated break-through profiles for the D,L-Met are presented. Here again the same effect for the first plateau with the D-enantiomer can be seen.
 |
Fig. (3) Comparison between experimental (solid lines) and simulated (dash lines) breack-through concentraion profiles for D,L-Met. |
The retention times and the slopes for the L-plateau are given properly, however a deviation in the height is observed as discussed above. Similar to D,L-Leu is also the situation with analytical injection (see Fig. ((4 )).
)).
 |
Fig. (4) Comparison between experimental (solid lines) and simulated (dash lines) concentraion profiles for D,L-Met (3.3 mmol L-1). |
Despite of the base line drift it can be seen, that retention times and the front part of the peaks were well predicted, but not the elution part. This is not surprising as during the adsorption isotherms measurements the desorption method could not be applied, as both enantiomers elute in a single desorption plateau. The reason is the complex interactions between the diastereomeric complexes, free amino acids, selector-copper complex and stationary phase.
In Fig. (5 )a comparison between experimental and simulated break-through data is given for D,L-Tyr. In contrast to the other two amino acids, D,L-Tyr is a hydrophobic one and the diastereomeric complexes interact stronger with the stationary phase leading to bigger capacity factors. Here compared to D,L-Leu and D,L-Met a good prediction for both plateaus is given by the model, but only at low concentrations. The deviation of the slopes increased with the increase in concentration. The small deviations in the height are due to calibration inaccuracy and base line drift. Having a look at Fig. (6
)a comparison between experimental and simulated break-through data is given for D,L-Tyr. In contrast to the other two amino acids, D,L-Tyr is a hydrophobic one and the diastereomeric complexes interact stronger with the stationary phase leading to bigger capacity factors. Here compared to D,L-Leu and D,L-Met a good prediction for both plateaus is given by the model, but only at low concentrations. The deviation of the slopes increased with the increase in concentration. The small deviations in the height are due to calibration inaccuracy and base line drift. Having a look at Fig. (6 ) one can see that both band profiles of D,L-Tyr are excellent predicted by the model. A very small deviation is observed only for the L-enantiomer.
) one can see that both band profiles of D,L-Tyr are excellent predicted by the model. A very small deviation is observed only for the L-enantiomer.
 |
Fig. (5) Comparison between experimental (solid lines) and simulated (dash lines) breack-through concentraion profiles for D,L-Tyr. |
 |
Fig. (6) Comparison between experimental (solid lines) and simulated (dash lines) concentraion profiles for D,L-Tyr (1.8 mmol L-1). |
3.4. Dual Chiral LEC System
As expected the deviations between experimental and calculated data for the dual chiral LEC system is bigger compared to the single one. This is due to the fact that two selectors simultaneously build complexes with the amino acids enantiomers. During loading experiments and adsorption isotherms measurements an interesting effect was observed, namely that the retention time for both enantiomers increased only for the L-one when applying the dual system, which reveals a complex adsorption behavior [13]. Furthermore, when applying the ED model for the elution band profiles a discretization three times finer compared to the calculations with the single one was necessary in order to achieve appropriate simulations.
In Fig. (7 ) the break-through curves at different feed concentrations for D,L-Leu together with the calculated profiles are shown. For all the concentrations, the predicted D-plateau is in very good agreement with the experimental one. The deviation is observed for the L-plateau. The model predicts faster elution of the L-enantiomer than the experiment. This confirms the observation during the loading experiments, that the L-enantiomer interacts stronger with the stationary phase than the D-enantiomer. Furthermore, displacement effects at higher concentrations are also observed, which is also reported in the literature for chiral systems [20, 21].
) the break-through curves at different feed concentrations for D,L-Leu together with the calculated profiles are shown. For all the concentrations, the predicted D-plateau is in very good agreement with the experimental one. The deviation is observed for the L-plateau. The model predicts faster elution of the L-enantiomer than the experiment. This confirms the observation during the loading experiments, that the L-enantiomer interacts stronger with the stationary phase than the D-enantiomer. Furthermore, displacement effects at higher concentrations are also observed, which is also reported in the literature for chiral systems [20, 21].
 |
Fig. (7) Comparison between experimental (solid lines) and simulated (dash lines) breack-through concentraion profiles for D,L-Leu. |
Similar behavior is observed for D,L-Met (see Fig. (4 )). Here again a deviation for the second L-plateau is observed, whereas the L-enantiomer elutes faster than predicted. This confirms the assumption, that the more hydrophilic amino acids form preferably their diastereomeric complexes in the mobile phase and the interaction with the stationary phase is weaker. In the case of the more hydrophobic amino acid D,L-Tyr (see Fig. (9
)). Here again a deviation for the second L-plateau is observed, whereas the L-enantiomer elutes faster than predicted. This confirms the assumption, that the more hydrophilic amino acids form preferably their diastereomeric complexes in the mobile phase and the interaction with the stationary phase is weaker. In the case of the more hydrophobic amino acid D,L-Tyr (see Fig. (9 )) the deviation is observed for the first plateau. The deviation in the height is due to calibration errors or an base line drift. Here the model predicts a slower elution that the experiments resulting from a stronger interaction with the stationary phase. One can assume, that the L-enantiomer interacts so strongly with the stationary phase, that all adsorption sites are covered and this is why the D-enantiomer remains in the mobile phase.
)) the deviation is observed for the first plateau. The deviation in the height is due to calibration errors or an base line drift. Here the model predicts a slower elution that the experiments resulting from a stronger interaction with the stationary phase. One can assume, that the L-enantiomer interacts so strongly with the stationary phase, that all adsorption sites are covered and this is why the D-enantiomer remains in the mobile phase.
 |
Fig. (8) Comparison between experimental (solid lines) and simulated (dash lines) breack-through concentraion profiles for D,L-Met |
 |
Fig. (9) Comparison between experimental (solid lines) and simulated (dash lines) breack-through concentraion profiles for D,L-Tyr. |
Having a look at Fig. (10 ) and Fig. (11
) and Fig. (11 ) it can be seen that the best peak profile simulation was possible only for L-Leu and L-Met, where the retention times and the shape could be predicted perfectly. However, for both D-Leu and D-Met a bigger amount was predicted, which is due to the influence of the chiral additive in the mobile phase, as it binds the D-enantiomers in diastereomeric complexes. It is worth to mention, that in the front of the elution band of the first eluted enantiomer, the D-one the concentration of the second enantiomer, the L-one, is zero. In the mixed zone between the two bands and particularly in the area of the front shock of the second enantiomer, there is a strong effect of counter-current diffusion in the adsorbent particle, where the D-enantiomer is diffusing out of the particle while the L-enantiomer is diffusing into it. The two chiral selectors and the concurrence between both enantiomers control this process which is obviously difficult to be described by the ED model.
) it can be seen that the best peak profile simulation was possible only for L-Leu and L-Met, where the retention times and the shape could be predicted perfectly. However, for both D-Leu and D-Met a bigger amount was predicted, which is due to the influence of the chiral additive in the mobile phase, as it binds the D-enantiomers in diastereomeric complexes. It is worth to mention, that in the front of the elution band of the first eluted enantiomer, the D-one the concentration of the second enantiomer, the L-one, is zero. In the mixed zone between the two bands and particularly in the area of the front shock of the second enantiomer, there is a strong effect of counter-current diffusion in the adsorbent particle, where the D-enantiomer is diffusing out of the particle while the L-enantiomer is diffusing into it. The two chiral selectors and the concurrence between both enantiomers control this process which is obviously difficult to be described by the ED model.
 |
Fig. (10) Comparison between experimental (solid lines) and simulated (dash lines) concentraion profiles for D,L-Leu (4 mmol L-1). |
 |
Fig. (11) Comparison between experimental (solid lines) and simulated (dash lines) concentraion profiles for D,L-Met (4 mmol L-1). |
Generally, because isotherms supply more information than retention factors measured under linear conditions, they permit a more detailed study of retention mechanism in liquid chromatography. Furthermore, the competitive behavior between enantiomers can have surprising results when they have markedly different isotherms (for instance D,L-Tyr in the single chiral LEC system).
4. CONCLUSION
The competitive adsorption isotherm data for the amino acids enantiomers are modeled by the multi-component competitive Langmuir equation and used in the equilibrium dispersive method. The model was applied to predict the adsorption behavior (break-through curves and analytical injections) of three amino acids in single and dual chiral LEC systems. In the single system the D-enantiomer of all amino acids showed an anti-Langmuir behavior, whereas in the dual system all D-Met and D-Tyr proved to have an anti-Langmuir behavior, whereas all other amino acids enantiomers have Langmuir behavior.
The break-through profiles calculated by the model are in general in good agreement with the experimental data, whereas some deviations are observed due to the simultaneous interaction of two chiral selectors with opposite selectivity. In the single system for the hydrophilic amino acids (D,L-Leu and D,L-Met) a deformation of the first plateau, e.g. the D-one, was observed and could not be described properly by the model. In the dual system the model predicted faster elution and a higher adsorption quantity for the D-enantiomers. In the case of D,L-Tyr which is an aromatic, hydrophobic amino acid very good prediction of the elusion profiles in the single system was observed. In the dual system the model predicted smaller retention times for the D-plateaus of the break-through curves.
These results confirm, that the equilibrium dispersive method give a satisfactory results for separating relatively small molecules such as amino acids even in the presence of two chiral selectors.
ACKNOWLEDGEMENT
The authors would like to thank Max-Buchner Stiftung and Landesforschungsschwerpunkt NanoKat for financial support.
P.Dimitrova thanks Dipl.-Wirtsch.-Ing Rouven Weiler, Chair of Particle Process Engineering, Technical University Kaiserslautern, for his kindly assistance regarding the numerical simulations.


