- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

The Open Virology Journal
(Discontinued)
ISSN: 1874-3579 ― Volume 15, 2021
Influenza A H1N1pdm 2009 Virus in Paraguay: Nucleotide Point Mutations in Hemagglutinin and Neuraminidase Genes are not Associated with Drug Resistance
Emilio E Espínola*, 1, Alberto A Amarilla 1, 2, Magaly Martínez 1, Víctor H Aquino 2, Graciela Russomando 1
Abstract
Influenza virus is associated with upper respiratory tract infections. The fourth influenza pandemic was declared in 2009. The aim of this study was to determine the genetic variability of the 2009 H1N1 pandemic virus circulating in Paraguay. Nasal swabs were collected from 181 patients with flu symptoms managed at the Hospital of the Medical School in Asunción, Paraguay, between August and October 2009. Virus detection was carried out by real-time reverse transcription-polymerase chain reaction, followed by sequencing of the hemagglutinin and neuraminidase genes, and phylogenetic analysis. H1N1pdm09 was detected in 14.9% (27/181) of the suspected cases. Analysis of 13 samples showed that these viruses the clustered in a single genetic group. Neither the mutation related to exacerbation of disease (D239G in hemagglutinin) nor that related to antiviral resistance (H275Y in neuraminidase), both detected in neighboring countries, were found. This genetic analysis of H1N1pdm09 will help to understand the spread of the disease.
Article Information
Identifiers and Pagination:
Year: 2014Volume: 8
First Page: 9
Last Page: 13
Publisher Id: TOVJ-8-9
DOI: 10.2174/1874357901408010009
Article History:
Received Date: 13/6/2014Revision Received Date: 15/7/2014
Acceptance Date: 18/7/2014
Electronic publication date: 29 /9/2014
Collection year: 2014
open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
* Address correspondence to this author at the Rio de la Plata y Lagerenza, CP1120, Asunción, Paraguay; Tel: +595 21 424 520; Fax: +595 21 480 185; E-mail: emilioespinola@hotmail.com
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 13-6-2014 |
Original Manuscript | Influenza A H1N1pdm 2009 Virus in Paraguay: Nucleotide Point Mutations in Hemagglutinin and Neuraminidase Genes are not Associated with Drug Resistance | |
INTRODUCTION
Influenza A viruses, which infect a wide variety of mammals and birds, belong to the Orthomyxoviridae family. These enveloped viruses have a genome composed of eight segments of single-stranded, negative-sense RNA. Two antigenic proteins are anchored in the virus envelope: Hemagglutinin (HA) and Neuraminidase (NA). These proteins, which exhibit higher antigenic variability than the other virus proteins, are the main determinants of pathogenicity [1Knipe DM, Howley PM, Griffin DE, Lamb RA, Krug RM, Eds. Orthomyxoviridae The viruses and their replication Fields Virology. Lippincott Williams & Wilkins.: Philadelphia 2001; pp. 1487-531.]. Eighteen HA subtypes (H1-H18), and eleven NA subtypes (N1-N11) have been found [2Tong S, Zhu X, Li Y , et al. New world bats harbor diverse influenza A viruses. PLoS Pathog 2013; 9(10): e1003657.], and several combinations of these proteins due to genetic reassortment can be detected in nature [1Knipe DM, Howley PM, Griffin DE, Lamb RA, Krug RM, Eds. Orthomyxoviridae The viruses and their replication Fields Virology. Lippincott Williams & Wilkins.: Philadelphia 2001; pp. 1487-531.].
The fourth influenza pandemic (H1N1) was declared on June 11 (2009) [3WHO Pandemic (H1N1):2009 update 75. In: Accessed on January 1 2012 Available from http: //wwwwhoint. ], after the cases of 1918 (H1N1), 1957 (H2N2), and 1968 (H3N2) [1Knipe DM, Howley PM, Griffin DE, Lamb RA, Krug RM, Eds. Orthomyxoviridae The viruses and their replication Fields Virology. Lippincott Williams & Wilkins.: Philadelphia 2001; pp. 1487-531.]. It is thought that the new 2009 H1N1 pandemic virus (henceforth, H1N1pdm09) has emerged through at least four reassortment and transmission events among swine, avian and human H1N1 lineages, probably in Asia and North America [4Qu Y, Zhang R, Cui P , et al. Evolutionary genomics of the pandemic 2009, H1N1 influenza viruses (pH1N 1v). Virol J 2011; 8: e250.]. Particularly, the HA segment of H1N1pdm09 was originated from the American swine lineage, whereas the NA segment derives from the European swine lineage [5Babakir-Mina M, Dimonte S, Perno CF, Ciotti M. Origin of the 2009, Mexico influenza virus a comparative phylogenetic analysis of the principal external antigens and matrix protein. Arch Virol 2009; 154(8): 1349-52., 6Garten RJ, Davis CT, Russell CA , et al. Antigenic and genetic characteristics of swine-origin 2009, A(H1N1):influenza viruses circulating in humans. Science 2009; 325(5937): 197-201.].
In South America, information about H1N1pdm09 diversity is scarce. However, circulation of antiviral resistant strains has been reported in countries neighboring Paraguay. In Argentina, a study carried out in 2009 isolated six oseltamivir-resistant strains containing the NA H275Y mutation, from 262 cases with mild to severe forms of the disease; none of the mutations in HA or NA were related to fatal cases [7Barrero PR, Viegas M, Valinotto LE, Mistchenko AS. Genetic and phylogenetic analyses of influenza A H1N1pdm virus in Buenos Aires, Argentina. J Virol 2011; 85(2): 1058-66.]. In Brazil, another study carried out in 2009 found one oseltamivir-resistant strain out of 305 cases from a patient with scarce medical record [8Souza TM, Resende PC, Fintelman-Rodrigues N , et al. Detection of oseltamivir-resistant pandemic influenza A(H1N1)pdm2009, in Brazil can community transmission be ruled outκ. PLoS One 2013; 8(11): e80081.].
In Paraguay, the first confirmed case was declared on May 19, 2009 and by the end of that year, 8,284 H1N1pdm09 suspected cases were reported, including 987 confirmed cases and 46 deaths (May to December 2009) [9DGVS MSPyBS (Paraguay) Boletín semanal de situación epidemiológica. January 8 2010 Accesed on January 30 2010 Available from http: //wwwvigisaludgovpy ]. Approximately 50% of the suspected cases were reported in July 2009, 60% of whom were female, 30% ranged from 20 to 39 years of age, and 60% were inhabitants of the Central Department and Asunción. The severity of the disease, and mortality related to H1N1pdm09 infection were associated with the presence of pre-existing medical conditions, such as obesity, pregnancy, diabetes mellitus, and cardiovascular disease, as well as with being male, older than 60 years, and not vaccinated against seasonal influenza virus during 2009 [10Cabello A, von Horoch M, Ojeda A , et al. Factores asociados a mortalidad en la pandemia de influenza H1N1 2009, en Paraguay. Mem Inst Investig Cienc Salud 2011; 7(1): 5-12.].
The aim of this study was to determine the genetic variability of the H1N1pdm09 viruses circulating in the Central Department of Paraguay during the pandemic phase. Nasal swabs were obtained from 181 children and adults with clinical symptoms of influenza-like illness or severe acute respiratory infection (suspected cases), without data of antiviral treatment, managed at the Hospital of the Medical School, National University of Asunción (UNA), Paraguay, between August and October 2009 [10Cabello A, von Horoch M, Ojeda A , et al. Factores asociados a mortalidad en la pandemia de influenza H1N1 2009, en Paraguay. Mem Inst Investig Cienc Salud 2011; 7(1): 5-12.]. This Hospital provides medical care to low-income families residing in the Central Department, which has a population of around two million (~25% of the Paraguayan population). Samples were collected by the hospital personnel using a synthetic swab (Dacron) and stored in 2 mL of viral transport medium (0.5% BSA, 100 U/mL penicillin, 100 U/mL gentamicin, diluted in PBS). Samples were collected within two-five days of the appearance of symptoms. These samples were maintained at 4ºC for up to three days and then sent to the Molecular Biology Laboratory of the Instituto de Investigaciones en Ciencias de la Salud, UNA (IICS-UNA). The samples were fractionated and stored at -80ºC until analysis. Samples were codified to maintain confidentiality of patients.
Total RNA was extracted from 200 µL of sample with the AxyPrep Body Fluid Viral DNA/RNA Miniprep Kit (Axygen Biosciences, CA, USA), following the manufacturer’s recommendations, and then eluted in 60 µL of nuclease-free water.
Real time reverse transcription-polymerase chain reaction (RT-PCR) analysis was carried out following standard procedures [11WHO CDC protocol of realtime RTPCR for influenza A(H1N1). April 284 2009 Available from http: //wwwwhoint ]. Briefly, the reaction contained 5 µL of total RNA, 0.5 µL of primers and TaqMan probes (Influenza A 2009 H1N1 Assay Sets v1.0, Applied Biosystems, CA, USA), 0.5 µL of one-step enzymes (AgPath-ID One-Step RT-PCR Kit, Ambion, CA, USA), and 12.5 µL of 2X buffer in a final volume of 25 µL. Each sample was analyzed in four different tubes, depending on the amplified gene target: matrix (InfA, 106-bp), swine nucleoprotein (swInfA, 195-bp), swine HA type 1 (swH1, 116-bp), and human RNase P (internal control, 65-bp). A 7500 Real-Time PCR System (Applied Biosystems) was used. The mixture was incubated at 50ºC for 30 min, followed by incubation at 95ºC for 2 min, and 40 cycles of amplification, each consisting of
incubations at 95ºC for 15 sec and 55ºC for 30 sec. A sample was considered positive if both the InfA and the respective subtype (swInfA or swH1) reaction curves crossed the threshold (Ct) line within the first 40 cycles [11WHO CDC protocol of realtime RTPCR for influenza A(H1N1). April 284 2009 Available from http: //wwwwhoint ].
The HA and NA genes were amplified by RT-PCR. The reaction for cDNA synthesis contained 10 µL of total RNA, 200 ng of random primers (Invitrogen, USA), 0.25 mM dNTPs mix (Invitrogen), 80 U of RNAseOUT (Invitrogen), 200 U of M-MLV Reverse Transcriptase (Promega, USA), and 8 μL 5X buffer (250 mM Tris-HCl pH 8.3, 375 mM KCl, 15 mM MgCl2, 50 mM DTT), in a final volume of 40 µL. The mixture was incubated at 25ºC for 10 min, followed by incubation at 37ºC for 4 h, and a final incubation of 5 min at 85ºC.
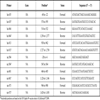
Three overlapping fragments of the HA and NA genes were amplified by PCR: HA (635-bp, 864-bp, and 813-bp), and NA (584-bp, 681-bp, and 473-bp). The amplification reaction contained 5 µL of cDNA, 0.15 µM of each primer (primers used are listed in Table 1), 0.25 mM dNTPs mix (Invitrogen), 1.5 U of DFS-Taq DNA polymerase (Bioron, Germany), and 5 µL 10X buffer II (500 mM KCl, 100 mM Tris-HCl pH 8.8, 0.1% Tween-20, 15 mM MgCl2), in a final volume of 50 µL. The cycling conditions were as follows: denaturation at 95ºC for 2 min, and 45 cycles of amplification, each consisting of denaturation at 95ºC for 30 sec, primer annealing at 45ºC for 30 sec, and primer extension at 72ºC for 5 min, followed by a final extension at 72ºC for 7 min. The PCR products were analyzed in 1.8% agarose gels, stained with ethidium bromide, and visualized under UV light. Standard procedures to avoid any type of contamination with amplicons were performed in different rooms.
The HA and NA PCR products were purified from 1.8% agarose gels, using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences), and directly sequenced (both strands, 3X coverage each) in an ABI PRISM 310 DNA analyzer (Applied Biosystems). Nucleotide sequences were manually edited with BioEdit v.7.0.5 [12Hall TA. BioEdit a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser 1999; 41: 95-8.], and aligned with
CLUSTAL W [13Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 1994; 22(22): 4673-80.]. The HA and NA sequences obtained were compared with 2,038 HA and 1,273 NA coding sequences of H1N1pdm09 reported worldwide during 2009-2010 [14Espinola EE. Genome stability of pandemic influenza A (H1N1):2009, based on analysis of hemagglutinin and neuraminidase genes. Open Virol J 2012; 6: 59-63.]. Phylogenetic relationships were reconstructed by the neighbor-joining method with Kimura’s two-parameter as the model of nucleotide substitution and bootstrap analysis of 1,000 replicates, as incorporated in MEGA v5 [15Tamura K, Peterson D, Peterson N , et al. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 2011; 28(10): 2731-9.]. The sequences selected from subtypes H1-H18 and N1-N11 were obtained from GenBank. The nucleotide sequences for the HA and NA genes obtained in this study were deposited in GenBank, under the following accession numbers: HA (JX625229– JX625241), and NA (JX625242– JX625254).
Written informed consent was obtained from parents or guardians of all participating individuals. This study was approved by the Ethics Committee of the IICS-UNA, under code M13/10.
Genomic RNA of H1N1pdm09 was detected by real-time RT-PCR in 14.9% (27/181) of the suspected cases. However, it was possible to amplify and sequence the DNA of the HA and NA genes of 13 samples, with Ct values<30. High percentage of genetic identity, ranging from 99.7% to 100% for the HA gene, and 99.5% to 100% for the NA gene, was observed among the viruses analyzed. This is in agreement with our previous report showing that the HA and NA gene sequences have a high percentage of identity with the H1N1pdm09 viruses reported worldwide during 2009-2010 [14Espinola EE. Genome stability of pandemic influenza A (H1N1):2009, based on analysis of hemagglutinin and neuraminidase genes. Open Virol J 2012; 6: 59-63.]. The high percentages of nucleotide identity are in agreement with the single clustering of Paraguayan samples and those from the 2009-2010 worldwide circulation, as shown in the phylogenetic trees (Fig. 1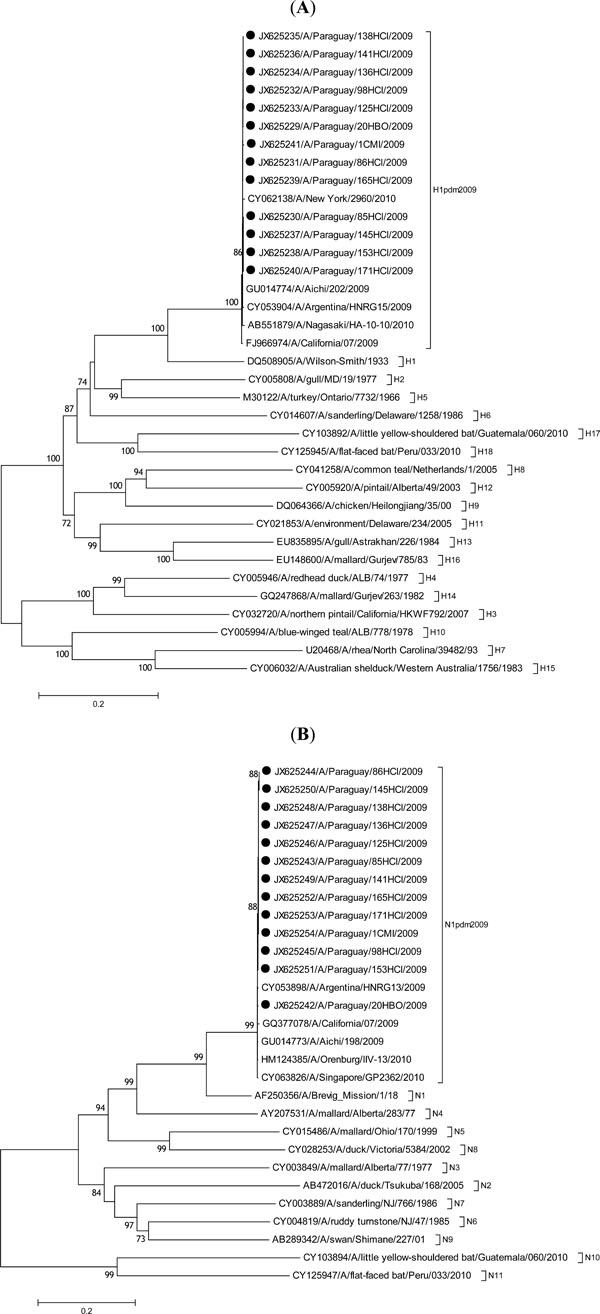 ). The high percentages of HA and NA nucleotide identity are also in agreement with published serological data, which show that the new pandemic viruses are antigenically very similar [6Garten RJ, Davis CT, Russell CA , et al. Antigenic and genetic characteristics of swine-origin 2009, A(H1N1):influenza viruses circulating in humans. Science 2009; 325(5937): 197-201.].
). The high percentages of HA and NA nucleotide identity are also in agreement with published serological data, which show that the new pandemic viruses are antigenically very similar [6Garten RJ, Davis CT, Russell CA , et al. Antigenic and genetic characteristics of swine-origin 2009, A(H1N1):influenza viruses circulating in humans. Science 2009; 325(5937): 197-201.].
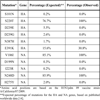
When the deduced HA and NA amino acid sequences of Paraguayan H1N1pdm09 viruses were compared with the early vaccine strain A/California/07/2009 (isolated in California, USA, and sequenced and published by the CDC on April 27) [16Centers for Disease Control and Prevention.Swine influenza A (H1N1):infection in two children-Southern California March-April 2009. MMWR Morb Mortal Wkly Rep 2009; 58(15): 400-2.], several amino acid mutations were found. Two amino acid substitutions, S220T (100%), and E391K (30.8%), were observed in the HA protein of the Paraguayan viruses (Table 2). The amino acid S220 is localized within the HA antigenic site designated Ca (site C, subsite a) as well as at the receptor binding domain (RBD); thus, S220T could affect the transmissibility and infectivity of H1N1 in humans. The substitution E391K found in this study has been previously identified as part of a highly conserved epitope in the 1918 H1N1 virus, with a possible role in membrane fusion [17Ekiert DC, Bhabha G, Elsliger MA , et al. Antibody recognition of a highly conserved influenza virus epitope. Science 2009; 324(5924): 246-51.]. In the HA protein, we did not find the S101N mutation, which is thought to be an adaptation to the human host, or D239E/G, which has been associated with severe clinical outcomes [18Kilander A, Rykkvin R, Dudman S, Hungnes O. Observed association between the HA1 mutation D222G in the 2009 pandemic influenza A(H1N1):virus and severe clinical outcome, Norway 2009-2010. Euro Surveill 2010; 15: e19498.] and exacerbate forms of respiratory disease [19Liu Y, Childs RA, Matrosovich T , et al. Altered receptor specificity and cell tropism of D222G hemagglutinin mutants isolated from fatal cases of pandemic A(H1N1):2009 influenza virus. J Virol 2010; 84(22): 12069-74.], or N387H, localized at a glycosylation site that could potentially affect the antigenic properties of influenza viruses [20Ikonen N, Haanpaa M, Ronkko E , et al. Genetic diversity of the 2009 pandemic influenza A(H1N1):viruses in Finland. PLoS One 2010; 5(10): e13329.].
The NA protein of the Paraguayan viruses showed two amino acid substitutions, V106I (100%) and N248D (100%). This is in agreement with the observation that both mutations were present in respiratory samples at increasing numbers through the early pandemic phase (April to December 2009)
[21Pan C, Cheung B, Tan S , et al. Genomic signature and mutation trend analysis of pandemic (H1N1):2009 influenza A virus. PLoS One 2010; 5(3): e9549.]. V106I was reported in H1N1 cases of the 20th century (in 1918 [pandemic] and 1977), whereas N248D was also reported in 1977. Since the residue at position 248 is located at the drug target domain (DTD), a mutation at this point could potentially affect the sensitivity of antiviral drugs. We did not find the NA D199N mutation associated with an increase in oseltamivir resistance published in both seasonal and H5N1 virus strains [22Deyde VM, Nguyen T, Bright RA , et al. Detection of molecular markers of antiviral resistance in influenza A (H5N1):viruses using a pyrosequencing method. Antimicrob Agents Chemother 2009; 53(3): 1039-47.], or the I223R mutation associated with resistance to oseltamivir, zanamivir and peramivir [23van der Vries E, Stelma FF, Boucher CA. Emergence of a multidrug-resistant pandemic influenza A (H1N1):virus. N Engl J Med 2010; 363(14): 1381-2.], or H275Y, located at the DTD and related to oseltamivir resistance especially in immunocompromised or severely ill people [24Harvala H, Gunson R, Simmonds P , et al. The emergence of oseltamivir-resistant pandemic influenza A (H1N1):2009 virus amongst hospitalised immunocompromised patients in Scotland, November-December, 2009. Euro Surveill 2010; 15(14): e19536.]. In countries neighboring Paraguay, such as Argentina and Brazil, however, some studies reported the circulation of oseltamivir-resistant strains [7Barrero PR, Viegas M, Valinotto LE, Mistchenko AS. Genetic and phylogenetic analyses of influenza A H1N1pdm virus in Buenos Aires, Argentina. J Virol 2011; 85(2): 1058-66., 8Souza TM, Resende PC, Fintelman-Rodrigues N , et al. Detection of oseltamivir-resistant pandemic influenza A(H1N1)pdm2009, in Brazil can community transmission be ruled outκ. PLoS One 2013; 8(11): e80081.]. Thus, we cannot discard the possibility of circulation of H1N1pdm09 drug-resistant strains during the pandemic phase in Paraguay. The lack of detection could be related to the small number of sequenced viruses from confirmed cases in the country during the study period.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
This work (project code: INV11) was supported by the Consejo Nacional de Ciencia y Tecnología (CONACYT) ― Programa de Apoyo al Desarrollo de la Ciencia, Tecnología e Innovación en Paraguay (BID 1698/OC-PR).