The Open Process Chemistry Journal
(Discontinued)
ISSN: 1875-1806 ― Volume 6, 2014
Antiproliferative Properties of Embelia ribes
Beena Joy*, a , Lakshmi S. b
Abstract
Embelia ribes is a threatened woody shrub belonging to the family Myrsinaceae, which is sparsely distributed in the moist deciduous forests of the Western Ghats in India.
The previous reports showed the effect of Embelin in methylcholanthrene induced fibrosarcoma in albino rats besides enhancing their survival time. Embelin provided a good support for the chemopreventive effect against N- nitrosodiethylamine/ phenobarbital induced hepatocarcinogenesis in Wistar rats. The present studies on cancer cell lines revealed that the crude hexane extract of the fruits of Embelia ribes exhibited cytotoxicity against Human leukaemic cells (K562) and Dalton’s Lymphoma ascites cells (DLA). Bioactivity directed fractionation and isolation yielded Embelin as the active principle. Invitro studies on Embelin suggest the potential of the compound on these two cell lines. However the compound did not exhibit toxicity on normal lymphocytes isolated from human blood preferentially attacking the tumour cells. This is the first report of invitro cytotoxic activity of Embelia ribes
Article Information
Identifiers and Pagination:
Year: 2010Volume: 3
First Page: 17
Last Page: 22
Publisher Id: TOCPCJ-3-17
DOI: 10.2174/1875180601003010017
Article History:
Received Date: 10/12/2009Revision Received Date: 15/11/2010
Acceptance Date: 16/11/2010
Electronic publication date: 31 /12/2010
Collection year: 2010
open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
* Address correspondence to this author at the National Institute for Interdisciplinary Science &Technology, Thiruvananthapuram- 695019, India; Tel: +91-471-2515354; Fax: +91-471-2491712; E-mail: bjoy39@yahoo.co.in
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 10-12-2009 |
Original Manuscript | Antiproliferative Properties of Embelia ribes | |
1. INTRODUCTION
Embelia ribes is a threatened woody shrub belonging to the family Myrsinaceae, which is sparsely distributed in the moist deciduous forests of the Western Ghats in India. In Indian system of medicine ‘Ayurveda’, the plant is popularly known as ‘Vidanga’. Embelin, a naturally occurring quinonoid compound, is found to be the major constituent of Embelia ribes [1].
Chemical structure of embelin (2, 5-dihydroxy-3-undecyl-1, 4-benzoquinone).
Embelin has a long alkyl chain (undecyl) as a substituent, which confers solubility in the non-polar phase and cell permeability. The adjacent quinone and hydroquinone groups on embelin form intramolecular hydrogen bonds. Embelin shows a wide spectrum of biological activities. The antibacterial activity of embelin was reported [2]. The antitumor, antiinflammatory and analgesic activity of embelin has been reported [3]. Embelin shows antitumour activity in methylcholanthrene induced fibrosarcoma in albino rats besides enhancing their survival time. The study of embelin suggests that, embelin can act as an antioxidant in physiological conditions [4].
2. MATERIALS AND METHODS
2.1. Isolation of Marker Compound Embelin and Purification
The hexane extract was subjected to column chromatography over silica gel (100-200 mesh). Elution of the column with benzene yielded an orange colour powder. The purification of embelin has been done by the crystallization using diethyl ether, yielded orange flakes of embelin. The spectral characterization of isolated compound was done, and was satisfied with the structure of embelin. The purity of embelin was confirmed by using HPLC and HPTLC analysis.
2.2. Cell Lines
Dalton’s lymphoma ascites (DLA) cells, Human Leukaemic cells (K- 562) cells, Lymphocytes isolated from human blood. Daltons Lymphoma Ascites (DLA) cells (arose as a spontaneous carcinoma of Thymus) were obtained from Cancer Instituite, Adayar.
DLA cells were propagated in the peritoneal cavity of mice by transplanting one million DLA cells per ml of Phosphate Buffered Saline (PBS). For experimental purposes, the tumour cells were aspirated from tumour bearing mice asceptically. K-562 (Human leukaemic cells) were obtained from National Centre for Cell Sciences, Pune, India and the cells were maintained in DMEM media supplemented with 10% FBS, 100 units/ml of Penicillin and 100 µg/ml Streptomycin in a humidified 5% CO2 incubator.
2.3. Experimental Animal maintenance
Balb/C mice (7-8 weeks old) weighing 20-25 gram were used for experiments. They were maintained on normal mouse chow (Lipton, India) and water ad libitum and housed in ventilated cages.
2.4. Reagents
Trypan blue, MTT (3-4,5-dimethylthiazol-2-yl2,5-diphenyltetrazolium bromide), Dulbecco’s modified eagle’s (DMEM) media, Fetal Bovine serum (FBS), Isopropanol, Dimethyl sulfoxide (DMSO), Sodium Dodecyl sulfate (SDS)
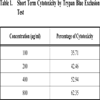
2.5. Short Term Cytotoxicity Studies
Short term cytotoxicity studies were done on DLA cells by Trypan blue exclusion method. Cells were aspirated from the peritoneal cavity of tumour bearing mice and washed in PBS twice and counted using a haemocytometer. 1 million cells were taken for cell cytotoxicity studies. Different concentrations of the compound was added to the cells and then made up to 1 ml with PBS. Cells were incubated for 3 hours at 37oC. After incubation, the cell death was evaluated using Trypan Blue exclusion method. To the cell suspension, 3 drops of Trypan Blue (0.5 % in PBS) was added and the cells were loaded immediately on to a haemocytometer. The number of Dead cells was counted and the percentage of dead cells was calculated. Viable cells exclude the dye while non viable cells take up the dye and appear blue in colour.
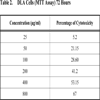
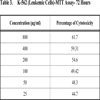
2.6. Cell Viability Assay by MTT
MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay is based on the ability of viable cells to reduce MTT from a yellow water-soluble dye to a dark blue insoluble formazan product [5].
5x103 cells were plated in 100 µl of the medium (DMEM with 10% FBS) in 96 well corning plates in the presence or absence of various concentrations of the compound for 48 and 72 hours [6]. Media treated cells was taken as negative control. At the end of the incubation, 25 µl of MTT solution (5 mg/ ml in PBS) was added to each well. After a 2 hour incubation, at 37oC, 100µl of the extraction buffer was added (20% Sodium Dodecyl Sulfate) in 50% Dimethylformamide. After a 4 hour incubation, the optical densities at 570 nm was taken using a multiwell plate reader with the extraction buffer as blank [7]. The minimum concentration required for 50% cytotoxicity and Inhibitory concentration required for 50% cytotoxicity (IC50) was calculated.
2.7. Isolation of Lymphocytes from whole Blood
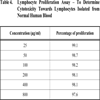
3ml of blood was taken in heparinised test tube. 5ml of PBS was added and mixed well by inversion. 3ml of ficoll hypaque solution was added in a conical centrifuge tube. Carefully layered 8ml of blood PBS mixture was added on to the ficoll hypaque. And then centrifuged at 2000 rpm for 30 minutes. The opaque interface containing mononuclear cells was aspirated and transferred into a clean conical centrifuge tube with a Pasteur pipette and discarded the upper layer. 10ml of PBS solution was added and mixed by inversion. Centrifuged at 1500rpm for 10 minutes and supernatant was discarded. Resuspended pellet with 5ml PBS and again centrifuged at 1500rpm for 10 minutes. Repeated the step thrice and resuspended lymphocyte pellet in 500µl PBS.
2.8. Determination of Cytotoxicity Towards Normal Lymphocytes
The cytotoxicity of the compound towards normal lymphocytes was determined by MTT assay described above.
2.9. Statistical Analysis
The data were expressed as mean± S.E.M. Results were analyzed statistically by one-way analysis of variance (ANOVA). P-value <0.05 was regarded as statistically significant.
3. RESULTS
The fraction containing embelin, the lead molecule of Embelia ribes exhibited cytotoxicity against DLA and K- 562 cells. Bioactivity directed fractionation led to the isolation of the lead molecule, Embelin. There is a graphical increase in the percentage of cytotoxicity with increasing time and concentration of the active compound. The compound exhibited 67% cytotoxicity on DLA cells at 800 µg/ml concentrations after 72 hours and 61.7% cytotoxicity on K-562 cells at the same time interval and concentration (Table 1,2, 3& 4). The Inhibitory concentration required for 50% cytotoxicity (IC50) was determined graphically. The IC50 of the active fraction against DLA cells and K-562 cells was 370 µg/ml and 114.5 µg/ml after 72 hours incubation (Graph 3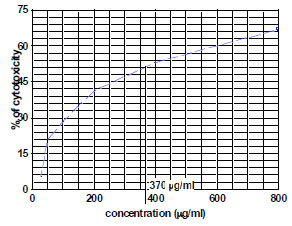 & 4
& 4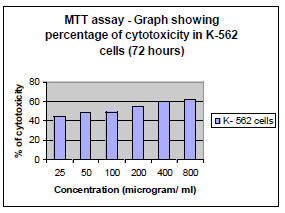 ). However the compound did not exhibit any cytotoxicity towards normal lymphocytes. The isolation, identification and quantification of embelin, the active principle of Embelia ribes had been investigated using HPLC, HPTLC, UV and different NMR techniques (Figs. 1
). However the compound did not exhibit any cytotoxicity towards normal lymphocytes. The isolation, identification and quantification of embelin, the active principle of Embelia ribes had been investigated using HPLC, HPTLC, UV and different NMR techniques (Figs. 1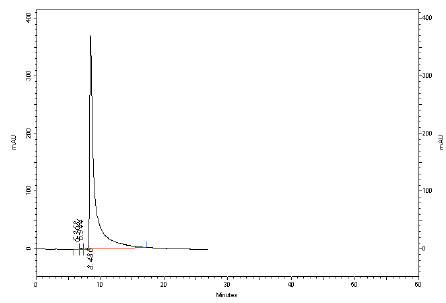 ,2
,2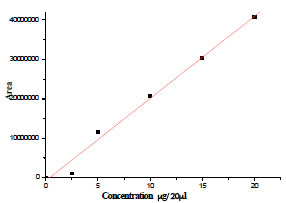 ,3
,3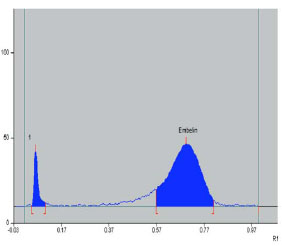 & 4
& 4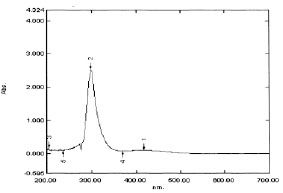 ).
).
 |
Graph. (3) Determination of IC50 on DLA cells by embelin after 72 hours |
 |
Graph. (4) |
 |
Fig. (1) HPLC Profile of isolated embelin at 298 nm. |
 |
Fig. (2) Calibration curve of embelin by HPLC. |
 |
Fig. (3) HPTLC Profile of isolated embelin at 298 nm. |
 |
Fig. (4) UV absorption spectrogram of embelin. |
Any Cancer therapy is basically aimed at killing the cancer cells while spuring the normal cells. Plants are continuously being screened throughout globe, in search of novel compounds that are more specific, toward eliminating tumour cells with least side effects. The survey of literature pertaining to Embelia ribes showed that investigations were carried out regarding its medicinal properties but few attempts had been made to investigate this plant, as a source of drug for cancer too. Further studies on the mechanism of action of the lead molecule on the cell cycle and apoptosis pathways are under progress.
4. DISCUSSION AND CONCLUSION
Invitrostudies on Embelin suggest the potential of the compound on the suspension cell lines DLA (Graph 1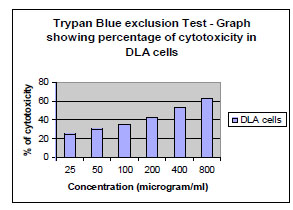 , 2
, 2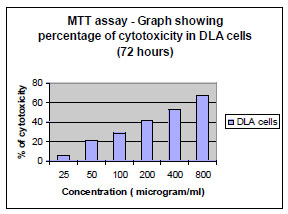 & 3
& 3 ) and K 562 (Table 3 & Graph 4
) and K 562 (Table 3 & Graph 4 ,5
,5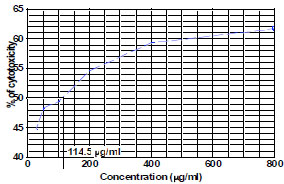 ). However the compound did not exhibit significant toxicity on normal lymphocytes (Table 4, Graph 6
). However the compound did not exhibit significant toxicity on normal lymphocytes (Table 4, Graph 6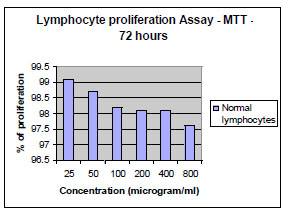 ) isolated from human blood preferentially attacking the tumour cells. The comparison of cytotoxicity (Graph 7
) isolated from human blood preferentially attacking the tumour cells. The comparison of cytotoxicity (Graph 7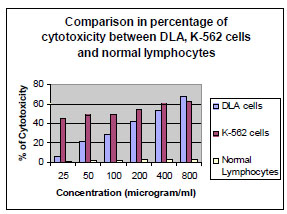 ) clearly indicated that this compound has no toxicity towards normal human cells. At the same time it is highly cytotoxic at 400µg/ml and 800µg/ml towards DLA and K-562 cell lines with IC 50 at 370 and 114.5µg/ml (Graph 4
) clearly indicated that this compound has no toxicity towards normal human cells. At the same time it is highly cytotoxic at 400µg/ml and 800µg/ml towards DLA and K-562 cell lines with IC 50 at 370 and 114.5µg/ml (Graph 4 , 5
, 5 , 6
, 6 & 7
& 7 ). The cytotoxicity for cyclophosphamide, the drug for the treatment of suspension type cancers is also evaluated invitro and and it is found that its cytotoxicity is high with that of embelin. But the toxicity towards normal lymphocytes are also high for the drug. The study indicated that embelin preferentially attack the cancer cell lines than cyclophosphamide This is the first report of invitro cytotoxic activity of Embelia ribes and its active principle embelin against DLA and K-562 cell lines.
). The cytotoxicity for cyclophosphamide, the drug for the treatment of suspension type cancers is also evaluated invitro and and it is found that its cytotoxicity is high with that of embelin. But the toxicity towards normal lymphocytes are also high for the drug. The study indicated that embelin preferentially attack the cancer cell lines than cyclophosphamide This is the first report of invitro cytotoxic activity of Embelia ribes and its active principle embelin against DLA and K-562 cell lines.
 |
Graph. (1) |
 |
Graph. (2) |
 |
Graph. (5) Determination of IC 50 on K-562 cells by embelin after 72 hours. |
 |
Graph. (6) |
 |
Graph. (7) Percentage of cell cytotoxicity-comparison between DLA, K-562 and Lymphocytes. |
5- O-ethylembelin and 5- O-methylembelin were reported to possess antiproliferative active against HL-60 leukemia cells and cervical carcinoma He La cells. Treatment with 10 microM of 1 and 2 for 24 h induced apoptosis in HL-60 cells. These compounds arrested HL-60 cells in the G(0)/G(1) phase of the cell cycle in a dose- and time-dependent manner. In HeLa cells, exposure to 100 microM of 1 or 2 for 6 h induced a complete disassembly of the microtubule network and an increased number of cells blocked in mitotic stages. This evidence suggests that both 1 and 2 are promising novel antimitotic and anticancer molecules targeting microtubular proteins [8]. Embelin induced caspase 3 and 9 activation in LNCaP and C4–2 cells by decreasing XIAP expression and was more potent than bicalutamide in killing prostate tumor cells irrespective of their androgen status [9]. Embelin is reported to be a fairly potent, nonpeptidic, cell-permeable, small-molecule inhibitor of XIAP and represents a promising lead compound for designing an entirely new class of anticancer agents that target the BIR3 domain of XIAP [10]. Embelin is reported to possess TRAIL induced apoptosis inducing activity in pancreatic cancer cells [11]. Our studies using colon cancer cells lacking p53 and Bax suggest that both lysosomes and mitochondria are prominent targets of embelin-induced cell death. Embelin induced cell-cycle arrest in G1 phase through p21, downstream of p53. In the absence of p21, the cells are sensitized to death in a Bax-dependent manner [12]. Our studies highlights the preliminary antiproliferative activity against Human Leukemic K-562 cells and Murine Lymphoma cells (DLA) for the first time and its relative non toxicity on normal human lymphocytes. More studies are being done in our lab in these Lymphoma as well as leukemic cells to evaluate its effects on the molecular mechanisms of apoptosis.
Our preliminary data of cytotoxic screening of different extracts of Embelia ribes showed hexane extract to be a promising one regarding its antitumour potential. The lead compound was also identified and it exhibited significant cytotoxicity towards tumour cells. As embelin is not soluble in water, we have made nanoembelin on biodegradable biopolymer to enhance the efficacy of the molecule in cancer therapy (Patent Submitted). And its antitumor activities invivo as well as invitro are in progress.
ACKNOWLEDGEMENTS
The authors wished to thank the Director, NIIST for financial support and Director Regional cancer center for extending the facilities to carry out the work.