The Open Bioactive Compounds Journal
(Discontinued)
ISSN: 1874-8473 ― Volume 9, 2020
Cytotoxic Activity-Guided Isolation from Achillea monocephala, and Biological Activities of its Different Extracts
Turgut Taşkın1, *, Eray M. Güler2, Şeyda Şentürk1, Damla D. Çelik3, Turan Arabacı4, Ümran S. Gürer3
Abstract
Background:
The genus Achillea is one of the most important genus of the Asteraceae family and many species of Achillea are used in traditional medicine to treat several ailments.
Aim:
The aim of the current research was to evaluate in vitro cytotoxic activities of n-hexan, chloroform, ethyl acetate and methanol extracts and to isolate the active compounds from the extract showing the strongest cytotoxic activity. In addition to this, it was aimed to evaluate the biological activities (cytotoxic, antioxidant, anti-urease, anticholinesterase, antimicrobial) of different extracts and active compounds from Achillea monocephala.
Methods and Materials:
The in vitro antioxidant, cytotoxic, anti-urease, anticholinesterase and antimicrobial activities of different extracts from A. monocephala aerial parts were examined. The structures of the active compounds were determined by NMR techniques, UV, IR and LC-MS/MS analysis and their biological potential was examined.
Results:
The chloroform extract showed strong and selective cytotoxic activity on the cancer cell lines (MDA-MB-231, MCF-7). Besides, this extract exhibited stronger antimicrobial activity than other extracts. Therefore, through activity-guided procedures, luteolin, naringenin and 8-hydroxy-salvigenin compounds were isolated from this extract. The methanol extract showed stronger antioxidant (DPPH, ABTS, CUPRAC) and anticholinesterase activity than other extracts. The n-hexan extract exhibited the highest anti-urease activity. In this study, it was determined that the isolated compounds had a strong biological activity. Naringenin compound had stronger ABTS radical cation scavenging and ferric reducing/antioxidant power, cytotoxic and antimicrobial activity than other compounds. 8-hydroxy-salvigenin compound showed the highest urease and acetylcholinestease enzyme inhibition.
Conclusion:
The results of this study suggest that the extracts and isolated compounds from the A. monocephala may be used as antioxidant, cytotoxic, anti-urease, anticholinesterase and antimicrobial agents in the future.
Article Information
Identifiers and Pagination:
Year: 2020Volume: 8
First Page: 7
Last Page: 14
Publisher Id: TOBCJ-8-7
DOI: 10.2174/1874847302008010007
Article History:
Received Date: 07/04/2020Revision Received Date: 18/05/2020
Acceptance Date: 18/05/2020
Electronic publication date: 18/08/2020
Collection year: 2020
open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: (https://creativecommons.org/licenses/by/4.0/legalcode). This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
* Address correspondence to this author at the Department of Pharmacognosy, Faculty of Pharmacy, Marmara University, Istanbul, Turkey; Tel: 90-506-306-9982; E-mails: turguttaskin@marmara.edu.tr,
ttaskin237@gmail.com
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 07-04-2020 |
Original Manuscript | Cytotoxic Activity-Guided Isolation from Achillea monocephala, and Biological Activities of its Different Extracts | |
1. INTRODUCTION
The genus Achillea is one of the most important genus of the Asteraceae family and has more than 100 species that grow wild in different regions of the world. The main habitats of this genus are concentrated in different parts of Iran, Turkey, Serbia and Eastern regions of Europe. The phytochemical studies of Achillea species reveal that this genus contains many components (flavones, guaianolides, lignans, non-saturated carboxylic acids, phenolic glycosides, phthalate derivatives, piperidine amides, polyacetylenes, proazulenes, sesquiterpene lactone diol, sesquiterpene lactones, sesquiterpenes, spirodepressolide, tannins, triterpene alkamides). Since the genus Achillea is widespread all over the world, many species of this genus have been used as traditional herbal medicines by the local population. It is known that there are many reports highlighting the ethnopharmacological uses of different species of Achillea. Many species of Achillea have been used as tonic, anti-inflammatory, anti-spasmodic, diaphoretic, diuretic and emmenagogic agents, wound healing and carminative. Achillea species are among the most important indigenous economic plants of Anatolia. Herbal teas prepared from some species of this genus are traditionally used in the treatment of abdominal pain and flatulence in Turkey [1Hosseini MM, Sarker SD, Akbarzadeh A. Chemical composition of theessential oils and extracts of Achillea species and their biological activities: a review. J Ethnopharmacol 2017; 199: 315-20.-3Küpeli E, Orhan I, Küsmenoglu S, Yesilada E. Evaluatüon of Antiinflammatory and antinociceptive activity of five anatolian Achillea species. Turk J Pharm Sci 2007; 4: 99-102.]. Globally cancer is one of the diseases that has seriously affected the human population. In recent years, natural compounds which are thought to have less toxic side effects than current treatments have attracted the attention of researchers. The number of studies on the use of compounds and extracts isolated from medicinal plants instead of synthetic compounds has increased in recent years [4Greenwell M, Rahman PKSM. Medicinal Plants: Their use in anticancer treatment. Int J Pharm Sci Res 2015; 6(10): 4103-12.
[PMID: 26594645] -6Janjušević L, Pejin B, Kaišarević S, et al. Trametes versicolor ethanol extract, a promising candidate for health-promoting food supplement. Nat Prod Res 2018; 32(8): 963-7.
[http://dx.doi.org/10.1080/14786419.2017.1366484] [PMID: 28817965] ]. To The best of our knowledge, there no reports on cytotoxic guided substance isolation of endemic Achillea monocephala. Hence, the aim of the current research was to evaluate in vitro cytotoxic activities of n-hexane, chloroform, ethyl acetate and methanol extracts and to isolate the active compounds from the extract showing the strongest cytotoxic activity. In addition to this, it was aimed to evaluate the biological activities (cytotoxic, antioxidant, anti-urease, anticholinesterase, antimicrobial) of the different extracts and active compounds from the plant.
2. MATERIALS AND METHODS
2.1. Chemical Reagents
DPPH (2,2-diphenyl-1-picrylhydrazyl), Folin –Ciocalteu (FCR), ascorbic acid, acetylcholinesterase (AChE), galantamine were obtained from Sigma Chemical Co. (Sigma-Aldrich GmbH, Sternheim, Germany). All other chemicals and solvents were of analytical grade. The column chromatography (CC) was achieved by using silica gel 0.2-0.5-mm (Sigma-Aldrich, USA) as stationary phases. Kieselgel 60 F254 (Merck) precoated plates were used for thin layer chromatography. Fetal bovine serum (FBS), Leibovitz's L15 Medium, McCoy’s 5a Medium, RPMI 1640 Medium, Eagle's Minimum Essential Medium (E’MEM), penicillin-streptomycin, cholera toxin, and transferrin were obtained from Sigma-Aldrich (Seelze, Germany). MEGM Kit was obtained from Lonza/Clonetics Corporation (Basel, Switzerland).
2.2. Spectroscopic Methods
The NMR spectroscopic analyses were performed on a Bruker Avance III (500 MHz). MS analyses were performed using Q-TOF-LC/MS (Agilent6530, CA, US). Shimadzu UV-1800 and FT-IR Affinity-1 spectrophotometer was used for UV and IR spectra, respectively.
2.3. Plant Material and Preparation of Plant Extracts
The aerial parts of Achillea monocephala Boiss. et Bal. were authenticated by Prof. Dr. Turan Arabacı. A voucher specimen (TA3042) was deposited at the herbarium of the Faculty of Pharmacy, İnönü University, for future reference.
2.4. Extraction and Isolation
The plant aerial parts were dried in shade (25°C) and grounded in a mechanic grinder (Renas, RBT1250) to a fine powder. The powdered sample (350 g) was extracted with organic solvents such as n-hexan, chloroform, ethyl acetate and methanol using the maceration method, respectively, until colorless. The organic phase was evaporated to dryness under reduced pressure. All the extracts obtained were stored at 4 °C for future analysis. The chloroform subextract (8 g) was submitted to silica gel column and eluted with n-hexan, chloroform and methanol mixture of increasing polarity, n-hexan (100%, 700 mL), n-hexan: chloroform (30%, 50%, 2x500 mL), chloroform (100%, 700 mL), chloroform: methanol (30%, 50%, 2x500 mL), methanol (100%, 500 mL), to obtain thirteen fractions. After these sub-fractions were controlled by TLC, similar ones were combined (Fr.I-V). Fr. I (0.40 g) was applied to a silica gel column using mixtures of toluene: ethyl acetate (7:3) to yield luteolin (4.3 mg). Fr. III. was applied to preparative TLC using mixtures of toluene: ethyl acetate: methanol (70:30:10) to yield naringenin (6.2 mg). Fr. V was applied to a silica gel column using mixtures of toluene: ethyl acetate (80:20; 70:30, 50:50) and ethyl acetate: methanol (80:20; 70:30, 50:50) to yield 8-hydroxy-salvigenin (5.1 mg).
2.5. Cytotoxic Activity of Different Extracts from Plant
The cell culture and maintenance: MDA-MB-231 cells (as standard cell line from human breast carcinoma cells), MCF-7 cells (as standard cell line from human breast carcinoma cells) and 184A1 (as standard human normal breast/epithelium cells) were obtained from American Type Cell Culture Collection (ATCC, Germany). MDA-MB-231 cells were cultured in Leibovitz's L15 Medium, MCF-7 cells were cultured in E’MEM and 184A1 cells were cultured in MEBM. The medium was supplemented with 10% FBS, 100 U/mL of penicillin and 100 ng/mL of streptomycin. Trypan blue exclusion test was used for the estimation of the number of viable cells.
Cytotoxicity assay: The cytotoxic activity of extracts and isolated compounds on the cells were examined by ATP levels measured with luminescence test (Cell-Titer-Glo Luminescent Cell Viability Assay, Promega). Cells were seeded onto 96-well plates at a density of 1.5×104 cells per well and incubated overnight at 37ºC in 5% CO2. The medium was then changed with a fresh complete medium including varying concentrations of extracts and isolated compounds. Control cells were treated with 0.1% DMSO. Cells were left for incubation under humidified 5% CO2 and 95% O2 at 37ºC for 24 hours. Afterward, the cells were rinsed with the culture medium and examined for ATP. Each sample was supplemented with 100 µL of the reagent (Cell Titer-Glo Luminescent Cell Viability Assay, Promega), mixed for 2 minutes and incubated for 10 minutes at room temperature. The results were evaluated with a luminometer (Varioscan Flash Multimode Reader, Thermo, Waltham, MA). The light emitted in the presence of ATP was measured as relative light units (RLU). The intensity of the emitted light quantities is directly related to the ATP content in the sample being tested. Cell viability was expressed as a percentage relative to the negative control group indicated as 100%. Half-maximal growth inhibitory concentration (IC50) values were calculated from non-linear regression analysis from concentration-response curves. Live/dead assay was performed to quantify live and dead cells as described previously. All experiments were repeated three times and the standard deviation was 5% [7Tuncay S, Senol H, Güler EM, et al. Synthesis of oleanolic acid analogues and their cytotoxic effects on 3T3 Cell Line. Med Chem 2018; 14(6): 617-25.
[http://dx.doi.org/10.2174/1573406414666180222094544] [PMID: 29473521] ].
2.6. Antioxidant Study
2.6.1. DPPH Radical Scavenging Assay
240 µL 0.1 mM DPPH solution was added to 10 µL sample of the extracts and active compounds. The prepared mixture was stirred for 1 min. and placed at 25ºC for 30 min. The mixture absorbance was determined against the reference at 517 nm. The control sample was carried out under the same conditions using 10 µL of methanol instead of experimental and standard materials and the control sample was daily measured. The investigation was performed three times and the averages of the data and standard deviation were calculated. The data were given as IC50=mg/mL [8Wei FC, Jinglou C, Yongfang C, Liming PL. Antioxidant, free radical scavenging, anti-inflammatoryand hepatoprotective potential of the extract from Parathelypteris nipponica (Franch. etSav.) Ching. J Ethnopharmacol 2010; 130: 528-35.].
2.6.2. ABTS Radical Cation Scavenging Assay
40 μL of extracts/active compunds were prepared and then 3960 μL of ABTS.+ working solution was added on the prepared mixer. The mixture absorbance was determined against the reference at 734 nm for 6 min. The control sample was prepared under the same conditions with the use of 40 µL distilled water instead of experimental and standard materials. The control sample was measured daily. ABTS radical scavenging determination was applied to trolox solutions prepared at different concentrations (0.2-1 mM). The results of this study were given as mM trolox/mg extract [9Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 1999; 26(9-10): 1231-7.
[http://dx.doi.org/10.1016/S0891-5849(98)00315-3] [PMID: 10381194] ].
2.6.3. Ferric Reducing/Antioxidant Power (FRAP) Assay
The method of Benzie and Strain (1996) was applied to the extracts/active compounds in order to estimate the ferric reducing ability. The FRAP reagent [25 mL 300 mM acetate buffer (pH 3.6), 2.5 mL of TPTZ solution and 2.5 mL 20 mM FeCl3·6H2O] was kept at 37ºC for 30 min. 190 µL FRAP reagent was mixed with 10 µL extract and the mixture absorbance was determined at 593 nm after 4 min. FRAP values of the extracts were given as mM Fe2+/mg extract [10Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 1996; 239(1): 70-6.
[http://dx.doi.org/10.1006/abio.1996.0292] [PMID: 8660627] ].
2.6.4. Cupric Ion Reducing/Antioxidant Power (CUPRAC) Assay
Briefly, 60 µL Cu(II)x2H2O, 60 µL neocuproine and 60 µL, NH4Ac (1 M) were mixed. Then, 60 µL of the extracts/active compounds and 10 µL of ethanol were added to the mixture. After the duration time of 60 min, the mixture absorbance was spectrophotometrically measured at 450 nm. CUPRAC values of the extracts were given as mM trolox/mg extract [11Apak R, Güçlü K, Ozyürek M, Karademir SE. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J Agric Food Chem 2004; 52(26): 7970-81.
[http://dx.doi.org/10.1021/jf048741x] [PMID: 15612784] ].
2.6.5. Total Phenolic Contents
Total phenolic content of plant extracts was determined with the Folin-Ciocalteu reagent (FCR) method. Briefly, 0.1 mL of the extract (5-0.5 mg/mL) was put in a plate and 4.5 mL of water was added. Then, 0.1 mL of the Folin-Ciocalteu reagent (diluted 1:3 with distilled water) and 0.3 mL of 2% sodium carbonate solution were added to the mixture. The mixture was left at room temperature for 2 h, and then absorbance was measured against the reference at 760 nm. Total phenolic contents were expressed as mg of gallic acid equivalents per mg of the extract [12Taskin T, Çam ME, Taşkın D, Rayaman E. In vitro and In vivo biological activities and phenolic characterization of Thymus praecox subsp. skorpilii var. skorpilii. J Food Meas Charact 2019; 13: 544-8.
[http://dx.doi.org/10.1007/s11694-018-9967-1] ].
2.7. Anti-urease Activity Assay
Briefly, working solution (100 µL) was taken and then urease (500 µL) was added on it. The mixture was incubated at 37ºC for 30 min. Then, 1100 µL of urea was added to this mixture and kept in the incubator at 37ºC for 30 min. R1 (1% phenol, 0.005% sodium nitroprusside) and R2 (0.5% NaOH, 0.1% sodium hypochlorite) reagents were added to the mixture, respectively. After the incubation period at 37ºC for 2 h, the absorbance of samples was measured at 635 nm [13Ghous T, Akhtar K, Nasim FUH, Choudhry MA. Screening of selected medicinal plants for urease inhibitory activity. Biol Med (Aligarh) 2010; 2: 69-73.].
2.8. Anticholinesterase Activity Assay
Briefly, AChE (20 µL) and different concentrations of extracts (20 µL) were added to phosphate buffer solution (pH 8.20, 0.1 M, 40 µL). This mixture was incubated at 25ºC for 10 min. After incubation, DTNB (100 μL) and AcI (20 μL) as substrate were added to the mixture. The same procedure was applied to the galantamine used as standard. The 5-thio-2-nitrobenzoic acid was spectrophotometrically measured at 412 nm [14Ellman GL, Courtney KD, Andres V Jr, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 1961; 7: 88-95.
[http://dx.doi.org/10.1016/0006-2952(61)90145-9] [PMID: 13726518] ].
2.9. Antimicrobial Activity
The antimicrobial activities of the extracts and compounds were examined by microbroth dilution method against Staphylococcus aureus ATCC 25923, Staphylococcus epidermidis ATCC 12228, Escherichia coli ATCC 25922, Klebsiella pneumoniae ATCC 4352, Proteus vulgaris ATCC 13315, Pseudomonas aeruginosa ATCC 27853, Candida albicans ATCC 90028, Candida glabrata ATCC 90030, Candida guilliermondii KUEN 998, Candida tropicalis KUEN 1021, Candida parapsilosis ATCC 90018 and Candida krusei ATCC 6258 [15Clinical Laboratory Standards Institute (CLSI). Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard-third edition; CLSI document M27-A3 ,Wayne 2008., 16Clinical Laboratory Standards Institute (CLSI). Performance Standarts for Antimicrobial Susceptibility Testing; Twenty-First Informational Supplement 2011; 31(1) M100-S21].
2.10. Statistical Analysis
All the experiments were performed in triplicates. All data from the study were demonstrated as mean ± SD and analysed by the Graphpad Prism 5. Statistical differences between the study groups were analysed using two-way analysis of variance (ANOVA) followed by Tukey’s Multiple Comparison Test. Mean values were considered statistically significant when p<0.05.
3. RESULTS
3.1. Cytotoxic Activity of Different Extracts and Active Compounds from Plant
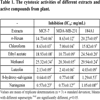
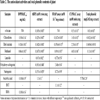
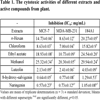
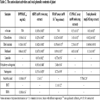
The cytotoxic activities of the n-hexan, chloroform, ethyl acetate and methanol extracts and active compounds on breast adenocarcinoma cell (MCF-7), breast adenocarcinoma cell receptor triple negative (MDA-MB-231) and healthy breast cell (184A1) were examined and results `given in Table 1. According to the results obtained from this study, it was determined that chloroform extract showed strong and selective cytotoxic activity against MDA-MB-231 (IC50: 7.00 mg/mL) and MCF-7 (IC50: 8.63 mg/mL) cell lines. It was also found that the methanol extract had low activity against MCF-7 (IC50: 25.32 mg/mL) and MDA-MB-231 (IC50: 20.30 mg/mL) cell lines. When the results of the extracts on the normal cell line (184A1) were evaluated, the cytotoxic activity of the chloroform extract on cancer cells (IC50: 7 mg/mL, 8.63 mg/mL) was found to be higher than in the normal cell line (IC50: 15.82). Therefore, cytotoxic activity-guided isolation was made from chloroform extract and luteolin, naringenin and 8-hydroxy-salvigenin were isolated as the active components. When cytotoxic activities of active compounds were evaluated, it was determined that all compounds (especially naringenin compound) showed stronger and selective cytotoxic activity on cancer cells (MCF-7, MDA-MB-231) than normal cell. Therefore, it is contemplated that these compounds may be used in the future as cytotoxic agents.
3.2. Structure Elucidation of the Active Components
The structures of the isolates were elucidated on the basis of their 1H NMR and HR-MS analysis and by comparison of their spectroscopic data with those published earlier.
3.2.1. 8-hydroxy-salvigenin
It was obtained as a yellow amorphous powder; C18H16O7, UV (MeOH) λmax: 214, 271, 336. IR (MeOH) υmax cm-1: 3316 (OH), 2943 (C-H), 2831, 1680 (C=O), 1447, 1411, 1261, 1180, 1113, 1025, 663. HR-MS: m/z =344.0872 g/mol, fragment ions: m/z =329.0555; 314.0340; 286.0370; 271.0223; 242.0228; 186.0310 g/mol. 1H NMR (500 MHz, CDCI3); δ3.78 (3H,s), δ3.83 (3H,s), δ3.97 (3H,s), δH: 8.00 (dd, J=3.5 Hz; J=9 Hz, H-2’/6’); δH: 6.96(dd, J=3.5 Hz; J=9.1 Hz, H-5’/3’); δH: 6.48(s, H-3); δH: 12.87 (s, H-5). These data were confirmed by the previous data [17Brahmachari G, Goraj D, Chatterjee D, Mondal S, Mistri B. 5,8 dihydoxy-6,7,4′-trimethoxyflavone, a novel flavonoid constituent of Limnophila indica. Indian J Chem 2004; 43: 222-6.-19Iqbal K, Iqbal J, Staerk D, Kongstad KT. Characterization of antileishmanial compounds from Lawsonia inermis L. Leaves using semi-high resolution antileishmanial profiling combined with HPLC-HRMS-SPE-NMR. Front Pharmacol 2017; 8: 337.
[http://dx.doi.org/10.3389/fphar.2017.00337] [PMID: 28620306] ].
3.2.2. Luteolin
It was obtained as a yellow amorphous powder. C15H10O6, HR-MS: m/z =285.0477 [M-H]+ g/mol; UVmax (MeOH) nm: 255, 348; IR υmax cm-1: 3208 (O-H), 1606 (C=O), 1508, 1442; 1H NMR (500 MHz, CDCI3); δ12.98 (1H,s, 5-OH), δ7.40 (H, dd, J=8.3, 2,1 Hz, H-6’), δ7.38 (1H,d J=2.5 Hz, H-2’), δ6.92 (1H,d J=8.3 Hz, H-5’), δ6.56 (1H,s, H-3), δ6.43 (1H,d J=2.3 Hz, H-8), δ6.24 (1H,d J=2.1 Hz, H-6). All data were identical with that of reported in literatüre [19Iqbal K, Iqbal J, Staerk D, Kongstad KT. Characterization of antileishmanial compounds from Lawsonia inermis L. Leaves using semi-high resolution antileishmanial profiling combined with HPLC-HRMS-SPE-NMR. Front Pharmacol 2017; 8: 337.
[http://dx.doi.org/10.3389/fphar.2017.00337] [PMID: 28620306] -21Rahate KP, Rajasekaran A. Isolation and Identification of flavone aglycones in roots of Desmostachya bipinnata Stapf. Indian J Pharm Sci 2018; 80: 556-9.
[http://dx.doi.org/10.4172/pharmaceutical-sciences.1000391] ].
3.2.3. Naringenin
C15H12O5, HR-MS: m/z =271.8542 [M-H]+ g/mol; UVmax (MeOH) nm: 288; IR υmax cm-1: 3271 (O-H), 1620 (C=O), 1590, 1500, 1083; 1H NMR (500 MHz, CDCI3); δ12.20 (1H,s, 5-OH), δ5.31 (H, dd, J=2.7, 12.99 Hz, H-2), δ3.12 (H,dd J=13.1, 17.00 Hz, H-3), δ2.69 (H,dd J=2.9, 17.00 Hz, H-3), δ5.92 (H,d J=2.3 Hz, H-6), δ5.91 (H,d, J=2.00, H-8), δ7.32 (H, dd, J=1.5, 8.2 Hz, H-2’/ H-6’), δ.6.85 (H,dd J=1.6, 8.3 Hz, H-3’/ H-5’). All data were identical with that of reported in literatüre (Fig. 1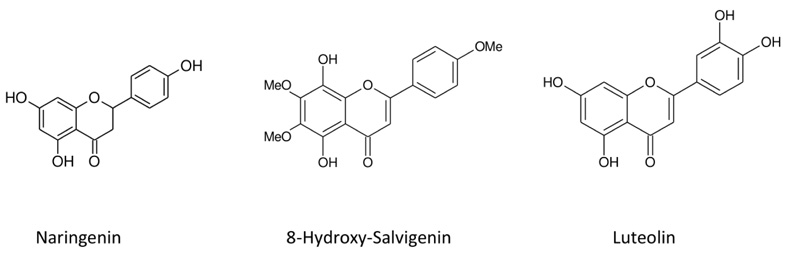 ) [22Guzel A, Aksit H, Elmastas M, Erenler R. Bioassay-guided isolation and identification of antioxidant flavonoids from Cyclotrichium origanifolium (Labill.) Manden and Scheng. Pharmacogn Mag 2017; 13(50): 316-20.
) [22Guzel A, Aksit H, Elmastas M, Erenler R. Bioassay-guided isolation and identification of antioxidant flavonoids from Cyclotrichium origanifolium (Labill.) Manden and Scheng. Pharmacogn Mag 2017; 13(50): 316-20.
[http://dx.doi.org/10.4103/0973-1296.204556] [PMID: 28539727] -24Cordenonsi LM, Sponchiado RM, Campanharo SC, Garcia CV, Raffin RP, Schapoval EES. Study of flavonoids presente in pomelo (Citrus máxima) by DSC, UV-VIS, IR, 1H AND 13C NMR and MS. Drug Anal Res 2017; 1: 31-6.
[http://dx.doi.org/10.22456/2527-2616.74097] ].
3.3. Biological Activities of Isolated Compounds and Different Extracts from Plant
3.3.1. Total Phenolic Contents
The total phenolic contents of different extracts were analyzed and presented in Table 2. When we compared the results obtained, it was found that methanol extract had the highest amount of phenolic contents.
 |
Fig. (1) Chemical structures of compounds isolated from plant. |
3.3.2. The Antioxidant Activity
The methanol extract fom plant showed stronger DPPH free radical scavenging (IC50: 0.06 mg/mL), ABTS radical cation scavenging (0.067 mM trolox/mg extract) and cupric reducing antioxidant (4.03 mM trolox/mg extract) activity than other extracts. In addition, chloroform extract exhibited the highest ferric reducing activity (0.167 mM Fe2+/mg extract). In this study, it was found that n-hexan extract showed no activity in the two methods (DPPH, FRAP) and the lowest activity in the other two methods (ABTS, CUPRAC). As shown in Table 2, the antioxidant activities of all extracts were lower than that of standard compounds (ascorbic acid, BHT, BHA). The antioxidant activities of the compounds isolated from the plant were examined using ABTS and FRAP methods and naringenin was found to have the strongest antioxidant activity. It was also determined that the naringenin and 8-hydroxy-salvigenin compounds had stronger ferric reducing activity than the standard BHT. When antioxidant activities of extracts and isolated compounds were compared, it was determined that isolated compounds had stronger ABTS radical cation scavenging and ferric reducing activity than extracts.
3.3.3. Urease and Acetylcholinesterase Inhibitory Activity
The results of anti-urease and anticholinesterase activity of different extracts and active compounds are shown in Table 3. The n-hexan extract (IC50: 0.023 mg/mL) exhibited the strongest anti-urease activity. In this study, it was also found that chloroform (IC50: 0.031 mg/mL) and ethyl acetate extracts (IC50: 0.030 mg/mL) had a very close anti-urease activity. Besides, the anti-urease activities of all extracts was shown to be lower than that of the standard Thiourea. When the anticholinesterase activities of plant extracts were compared, it was found that methanol extract (IC50: 0.018 mg/mL) had activity close to the standard compound (IC50: 0.012 mg/mL) and strong anticholinesterase activity than other extracts. When the activities of the isolated compounds on enzyme inhibition were evaluated, it was found that the 8-hydroxy-salvigenin compound had strong inhibition activity on both urease (IC50: 0.043 mg/mL) and acetylcholinesterase (IC50: 0.01 mg/mL) enzymes.
3.3.4. Antimicrobial Activity
In this study, the ethyl acetate extract showed antifungal activity against C. glabrata, C. krusei and C. parapsilosis while it did not show against bacteria. Similarly, the n-hexan extract showed antifungal activity against C. glabrata, C. guilliermondii, C. tropicalis, C. krusei and C. parapsilosis. In contrast, we did not find any effect on bacteria. The methanol extract showed antifungal activity against C. glabrata and C. krusei. The chloroform extract showed antibacterial activity against S.aureus and antifungal activity against all standard Candida species. The luteolin compound showed antifungal activity against C. albicans, C. guilliermondii, C. tropicalis, C.krusei and C. parapsilosis. The naringenin compound showed antibacterial activity against P. aeruginosa and antifungal activity against Candida species except C. glabrata. The 8-hydroxy-salvigenin compound showed antibacterial activity against P. vulgaris, P. aeruginosa, S. epidermidis and E. coli and antifungal activity against C. albicans, C.glabrata, C. guilliermondii, C.krusei and C. parapsilosis (Table 4).
4. DISCUSSION
A literature survey revealed that there are only two reports regarding the chemical contents and biological activity of A.monocephala. The contents of the essential oil obtained from the leaves and flowers of the plant were examined, borneol in the leaves and camphor, borneol, 1,8-cineole in the flowers were found to be major compounds [25Gogus F, Ozel MZ, Lewis AC. Extraction of essential oils of leaves and flowers of Achillea monocephala by superheated water. Flavour Fragrance J 2006; 21: 122-6.
[http://dx.doi.org/10.1002/ffj.1541] , 26Golmohammad F, Eikani MH, Maymandi HM. Cinnamon bark volatile oils separation and determination using solid-phase extraction and gas chromatography. Procedia Eng 2012; 42: 247-51.
[http://dx.doi.org/10.1016/j.proeng.2012.07.416] ]. In another study, the biological activities of ethanol extracts from root and aerial parts of the plant were investigated. In this study, it was observed that the root extract exhibited strong antioxidant and the aerial extract strong anticholinesterase and anti-urease activities. The chloroform: methanol (1:1, v/v) extract from plant’s root exhibited strong cytotoxic activity againts HeLa cell line. In addition, quinic acid, malic acid, fumaric acid, chlorogenic acid, vanillic acid, rutin, luteolin and apigenin compounds as major from root and aerial parts were analyzed using LC-MS/MS [27Yilmaz MA, Ertas A, Yener I, et al. A comprehensive LC-MS/MS method validation for the quantitative investigation of 37 fingerprint phytochemicals in Achillea species: A detailed examination of A. coarctata and A. monocephala. J Pharm Biomed Anal 2018; 154: 413-24.
[http://dx.doi.org/10.1016/j.jpba.2018.02.059] [PMID: 29602084] ]. In the present study, different from the above studies, biological activities (antioxidant, cytotoxic, anti-urease, anticholinesterase, antimicrobial) of different extracts (n-hexan, chloroform, ethyl acetate and methanol) from aerial parts of the plants were investigated. The active compounds (luteolin, 8-hydroxy-salvigenin, naringenin) were then isolated from the chloroform extract showing strong cytotoxic activity. Finally, the biological activities (antioxidant, cytotoxic, anti-urease, anticholinesterase, antimicrobial) of the isolated compounds were determined and the relationship between the active compounds and the biological activity was determined. The biological activity of luteolin and naringenin compounds isolated from the plant has been previously reported in several studies. The antioxidant (DPPH, ABTS) and anti-urease activity of luteolin compound were examined and it was found to have strong antioxidant and anti-urease activity [28Nile SH, Keum YS, Nile AS, Jalde SS, Patel RV. Antioxidant, anti-inflammatory, and enzyme inhibitory activity of natural plant flavonoids and their synthesized derivatives. J Biochem Mol Toxicol 2018; 32(1): 22-8.
[http://dx.doi.org/10.1002/jbt.22002] [PMID: 28972678] -32Hassan STS, Žemlička M. Plant-derived urease ınhibitors as alternative chemotherapeutic agents. Arch Pharm (Weinheim) 2016; 349(7): 507-22.
[http://dx.doi.org/10.1002/ardp.201500019] [PMID: 27244041] ]. It was also found that this compound has an cytotoxic effect on Huh-7, Hep2, HeLa, HepG2, MCF-7 and MDA-MB-231 cell lines [32Hassan STS, Žemlička M. Plant-derived urease ınhibitors as alternative chemotherapeutic agents. Arch Pharm (Weinheim) 2016; 349(7): 507-22.
[http://dx.doi.org/10.1002/ardp.201500019] [PMID: 27244041] -34Farid MM, Hussein SR, Ibrahim LF, et al. Cytotoxic activity and phytochemical analysis of Arum palaestinum Boiss. Asian Pac J Trop Biomed 2015; 1: 1-9.
[http://dx.doi.org/10.1016/j.apjtb.2015.07.019] ]. In our study, luteolin compound was found to have strong antioxidant, anti-urease and cytotoxic activity in parallel with the literature. Besides, this compound was found to have potent anticholinesterase and antifungal activity. The naringenin compound has been found to have antioxidant (DPPH, linoleic acid peroxidation, xanthine oxidase), antimicrobial and cytotoxic (SGC-7901, ABT-737 and HeLa cell lines) activities [35Lee EJ, Oh SY, Sung MK. Luteolin exerts anti-tumor activity through the suppression of epidermal growth factor receptor-mediated pathway in MDA-MB-231 ER-negative breast cancer cells. Food Chem Toxicol 2012; 50(11): 4136-43.
[http://dx.doi.org/10.1016/j.fct.2012.08.025] [PMID: 22926442] -39Krishnakumar N, Sulfikkarali N, Prasad NR, Karthikeyan S. Enhanced anticancer activity of naringenin-loaded nanoparticles in human cervical (HeLa) cancer cells. Biomedicine & Preventive Nutrition 2011; 1: 223-8.
[http://dx.doi.org/10.1016/j.bionut.2011.09.003] ]. When we compare the results of naringenin compound with the literature, it was observed that we obtained parallel results. In addition, naringenin compound was found to have potent anti-urease and anticholinesterase activities in this study.
CONCLUSION
The results of this study clearly demonstrated that the plant has potent antioxidant, cytotoxic, anti-urease, anticholinesterase and antimicrobial activities. The methanol extract was found to exert significant in vitro antioxidant and anticholinesterase activity. The chloroform extract showed the strongest cytotoxic and antimicrobial activity. The active components in chloroform were determined to be luteolin, naringenin, 8-hydroxy-salvigenin. The naringenin and 8-hydroxy-salvigenin compounds exhibited the strongest antioxidant, cytotoxic, antimicrobial, anti-urease and anticholinestease activity.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No animal/human were used in this research.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.
REFERENCES
| [1] | Hosseini MM, Sarker SD, Akbarzadeh A. Chemical composition of theessential oils and extracts of Achillea species and their biological activities: a review. J Ethnopharmacol 2017; 199: 315-20. |
| [2] | Saeidnia S, Gohari A, Mokhber-Dezfuli N, Kiuchi F. A review on phytochemistry and medicinal properties of the genus Achillea. Daru 2011; 19(3): 173-86. [PMID: 22615655] |
| [3] | Küpeli E, Orhan I, Küsmenoglu S, Yesilada E. Evaluatüon of Antiinflammatory and antinociceptive activity of five anatolian Achillea species. Turk J Pharm Sci 2007; 4: 99-102. |
| [4] | Greenwell M, Rahman PKSM. Medicinal Plants: Their use in anticancer treatment. Int J Pharm Sci Res 2015; 6(10): 4103-12. [PMID: 26594645] |
| [5] | Pejin B, Talevski A, Ciric A, et al. In vitro evaluation of antimicrobial activity of the freshwater sponge Ochridaspongia rotunda (Arndt, 1937). Nat Prod Res 2014; 28(18): 1489-94. [http://dx.doi.org/10.1080/14786419.2014.911297] [PMID: 24804931] |
| [6] | Janjušević L, Pejin B, Kaišarević S, et al. Trametes versicolor ethanol extract, a promising candidate for health-promoting food supplement. Nat Prod Res 2018; 32(8): 963-7. [http://dx.doi.org/10.1080/14786419.2017.1366484] [PMID: 28817965] |
| [7] | Tuncay S, Senol H, Güler EM, et al. Synthesis of oleanolic acid analogues and their cytotoxic effects on 3T3 Cell Line. Med Chem 2018; 14(6): 617-25. [http://dx.doi.org/10.2174/1573406414666180222094544] [PMID: 29473521] |
| [8] | Wei FC, Jinglou C, Yongfang C, Liming PL. Antioxidant, free radical scavenging, anti-inflammatoryand hepatoprotective potential of the extract from Parathelypteris nipponica (Franch. etSav.) Ching. J Ethnopharmacol 2010; 130: 528-35. |
| [9] | Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 1999; 26(9-10): 1231-7. [http://dx.doi.org/10.1016/S0891-5849(98)00315-3] [PMID: 10381194] |
| [10] | Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 1996; 239(1): 70-6. [http://dx.doi.org/10.1006/abio.1996.0292] [PMID: 8660627] |
| [11] | Apak R, Güçlü K, Ozyürek M, Karademir SE. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J Agric Food Chem 2004; 52(26): 7970-81. [http://dx.doi.org/10.1021/jf048741x] [PMID: 15612784] |
| [12] | Taskin T, Çam ME, Taşkın D, Rayaman E. In vitro and In vivo biological activities and phenolic characterization of Thymus praecox subsp. skorpilii var. skorpilii. J Food Meas Charact 2019; 13: 544-8. [http://dx.doi.org/10.1007/s11694-018-9967-1] |
| [13] | Ghous T, Akhtar K, Nasim FUH, Choudhry MA. Screening of selected medicinal plants for urease inhibitory activity. Biol Med (Aligarh) 2010; 2: 69-73. |
| [14] | Ellman GL, Courtney KD, Andres V Jr, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 1961; 7: 88-95. [http://dx.doi.org/10.1016/0006-2952(61)90145-9] [PMID: 13726518] |
| [15] | Clinical Laboratory Standards Institute (CLSI). Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard-third edition; CLSI document M27-A3 ,Wayne 2008. |
| [16] | Clinical Laboratory Standards Institute (CLSI). Performance Standarts for Antimicrobial Susceptibility Testing; Twenty-First Informational Supplement 2011; 31(1) M100-S21 |
| [17] | Brahmachari G, Goraj D, Chatterjee D, Mondal S, Mistri B. 5,8 dihydoxy-6,7,4′-trimethoxyflavone, a novel flavonoid constituent of Limnophila indica. Indian J Chem 2004; 43: 222-6. |
| [18] | Intekhab J, Aslam M. Isolation of a flavonoid from the roots of Citrus sinensis. Malays J Pharm Sci 2009; 7: 8-12. |
| [19] | Iqbal K, Iqbal J, Staerk D, Kongstad KT. Characterization of antileishmanial compounds from Lawsonia inermis L. Leaves using semi-high resolution antileishmanial profiling combined with HPLC-HRMS-SPE-NMR. Front Pharmacol 2017; 8: 337. [http://dx.doi.org/10.3389/fphar.2017.00337] [PMID: 28620306] |
| [20] | Tahira AW, Begüm S, Ayub A, et al. Luteolin and kaempferol from Cassıa alata, antimicrobial and antioxidant activity of iıts methanolıc extracts. FUUAST J Biology 2014; 4: 5-9. |
| [21] | Rahate KP, Rajasekaran A. Isolation and Identification of flavone aglycones in roots of Desmostachya bipinnata Stapf. Indian J Pharm Sci 2018; 80: 556-9. [http://dx.doi.org/10.4172/pharmaceutical-sciences.1000391] |
| [22] | Guzel A, Aksit H, Elmastas M, Erenler R. Bioassay-guided isolation and identification of antioxidant flavonoids from Cyclotrichium origanifolium (Labill.) Manden and Scheng. Pharmacogn Mag 2017; 13(50): 316-20. [http://dx.doi.org/10.4103/0973-1296.204556] [PMID: 28539727] |
| [23] | Jain R, Mittal M, Naringenın A. Flavonone from the stem of Nyctanthes arbortristis Linn. IJBPAS 2012; 1: 964-9. |
| [24] | Cordenonsi LM, Sponchiado RM, Campanharo SC, Garcia CV, Raffin RP, Schapoval EES. Study of flavonoids presente in pomelo (Citrus máxima) by DSC, UV-VIS, IR, 1H AND 13C NMR and MS. Drug Anal Res 2017; 1: 31-6. [http://dx.doi.org/10.22456/2527-2616.74097] |
| [25] | Gogus F, Ozel MZ, Lewis AC. Extraction of essential oils of leaves and flowers of Achillea monocephala by superheated water. Flavour Fragrance J 2006; 21: 122-6. [http://dx.doi.org/10.1002/ffj.1541] |
| [26] | Golmohammad F, Eikani MH, Maymandi HM. Cinnamon bark volatile oils separation and determination using solid-phase extraction and gas chromatography. Procedia Eng 2012; 42: 247-51. [http://dx.doi.org/10.1016/j.proeng.2012.07.416] |
| [27] | Yilmaz MA, Ertas A, Yener I, et al. A comprehensive LC-MS/MS method validation for the quantitative investigation of 37 fingerprint phytochemicals in Achillea species: A detailed examination of A. coarctata and A. monocephala. J Pharm Biomed Anal 2018; 154: 413-24. [http://dx.doi.org/10.1016/j.jpba.2018.02.059] [PMID: 29602084] |
| [28] | Nile SH, Keum YS, Nile AS, Jalde SS, Patel RV. Antioxidant, anti-inflammatory, and enzyme inhibitory activity of natural plant flavonoids and their synthesized derivatives. J Biochem Mol Toxicol 2018; 32(1): 22-8. [http://dx.doi.org/10.1002/jbt.22002] [PMID: 28972678] |
| [29] | Leung HWC, Kuo CL, Yang WH, Lin CH, Lee HZ. Antioxidant enzymes activity involvement in luteolin-induced human lung squamous carcinoma CH27 cell apoptosis. Eur J Pharmacol 2006; 534(1-3): 12-8. [http://dx.doi.org/10.1016/j.ejphar.2006.01.021] [PMID: 16469309] |
| [30] | Garcia OB, Castillo J, Lorente J, Ortun ÄA, Rio JAD. Antioxidant activity of phenolics extracted from Olea europaea L. Leaves. Food Chem 2000; 68: 457-61. [http://dx.doi.org/10.1016/S0308-8146(99)00221-6] |
| [31] | Mota KSL, Dias GEN, Pinto ME, et al. Flavonoids with gastroprotective activity. Molecules 2009; 14(3): 979-1012. [http://dx.doi.org/10.3390/molecules14030979] [PMID: 19305355] |
| [32] | Hassan STS, Žemlička M. Plant-derived urease ınhibitors as alternative chemotherapeutic agents. Arch Pharm (Weinheim) 2016; 349(7): 507-22. [http://dx.doi.org/10.1002/ardp.201500019] [PMID: 27244041] |
| [33] | Yoo DR, Jang YH, Jeon YK, et al. Proteomic identification of anti-cancer proteins in luteolin-treated human hepatoma Huh-7 cells. Cancer Lett 2009; 282(1): 48-54. [http://dx.doi.org/10.1016/j.canlet.2009.02.051] [PMID: 19342156] |
| [34] | Farid MM, Hussein SR, Ibrahim LF, et al. Cytotoxic activity and phytochemical analysis of Arum palaestinum Boiss. Asian Pac J Trop Biomed 2015; 1: 1-9. [http://dx.doi.org/10.1016/j.apjtb.2015.07.019] |
| [35] | Lee EJ, Oh SY, Sung MK. Luteolin exerts anti-tumor activity through the suppression of epidermal growth factor receptor-mediated pathway in MDA-MB-231 ER-negative breast cancer cells. Food Chem Toxicol 2012; 50(11): 4136-43. [http://dx.doi.org/10.1016/j.fct.2012.08.025] [PMID: 22926442] |
| [36] | Choi CW, Kim SC, Hwang SS, et al. Antioxidant activity and free radical scavenging capacity between Korean medicinal plants and flavonoids by assay-guided comparison. Plant Sci 2002; 163: 1161-5. [http://dx.doi.org/10.1016/S0168-9452(02)00332-1] |
| [37] | Kozłowska J, Potaniec B, Żarowska B, Anioł M. Synthesis and biological activity of novel o-alkyl derivatives of naringenin and their oximes. Molecules 2017; 22(9): 1485-92. [http://dx.doi.org/10.3390/molecules22091485] [PMID: 28878189] |
| [38] | Zhang H, Zhong X, Zhang X, Shang D, Zhou YI, Zhang C. Enhanced anticancer effect of ABT-737 in combination with naringenin on gastric cancer cells. Exp Ther Med 2016; 11(2): 669-73. [http://dx.doi.org/10.3892/etm.2015.2912] [PMID: 26893664] |
| [39] | Krishnakumar N, Sulfikkarali N, Prasad NR, Karthikeyan S. Enhanced anticancer activity of naringenin-loaded nanoparticles in human cervical (HeLa) cancer cells. Biomedicine & Preventive Nutrition 2011; 1: 223-8. [http://dx.doi.org/10.1016/j.bionut.2011.09.003] |