- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

Current Chemical Genomics and Translational Medicine
(Discontinued)
ISSN: 2213-9885 ― Volume 12, 2018
Healthy Adult LDL-C Bears Reverse Association with Serum IL-17A Levels
Azam Roohi1, *, Mina Tabrizi2, Mehdi Yaseri3, Fereshteh Mir Mohammadrezaei4, Behrouz Nikbin5
Abstract
Background:
Hypercholesterolemia is a modifiable risk factor in atherosclerosis with a complex association with inflammation.
Objective:
In the present study, the association between low-density lipoprotein cholesterol (LDL-C) and interleukin 17A (IL-17A), as an inflammatory cytokine, was investigated. In addition to IL-17A, serum levels of interleukin 23 (IL-23) and transforming growth factor β (TGF-β), as effective cytokines in T helper 17 cell (Th17) development, were also determined.
Method:
Cytokine levels were measured using enzyme-linked immunosorbent assay (ELISA) in healthy subjects with LDL-C<130 versus LDL-C=>130 mg/dL.
Results:
Although IL-17A is an inflammatory cytokine and a positive association between its levels and LDL-C is expected, the data obtained in this study provide support for a reverse association (p<0.05).
Conclusion:
Inflammation plays a major role in atherosclerosis development; however, various inflammatory components involved in atherosclerosis assert their own unique association with hypercholesterolemia.
Article Information
Identifiers and Pagination:
Year: 2018Volume: 12
First Page: 1
Last Page: 8
Publisher Id: CCGTM-12-1
DOI: 10.2174/2213988501812010001
Article History:
Received Date: 27/12/2017Revision Received Date: 26/05/2018
Acceptance Date: 12/06/2018
Electronic publication date: 29/06/2018
Collection year: 2018
open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: (https://creativecommons.org/licenses/by/4.0/legalcode). This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
* Address correspondence to this author at the Department of Immunology, School of Public Health, Tehran University of Medical Sciences, Tehran, 14716-13151, Iran, Tel: +98-21-42933149, Fax: +98-21-88954913; Email: aroohi@farabi.tums.ac.ir
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 27-12-2017 |
Original Manuscript | Healthy Adult LDL-C Bears Reverse Association with Serum IL-17A Levels | |
1. INTRODUCTION
Atherosclerosis is one of the most common causes of cardiovascular disease. It begins early in life, making primary prevention efforts necessary from childhood [1Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA 2001; 285(19): 2486-97.
[http://dx.doi.org/10.1001/jama.285.19.2486] [PMID: 11368702] ]. Thus, there is increasing emphasis on preventing atherosclerosis by modifying risk factors. Dyslipidemia is one of the nine potentially modifiable factors accounting for over 90% of the population-attributable risk for a first Myocardial Infarction (MI) as demonstrated by the INTERHEART study, the first study to systematically examine the determinants of vascular disease in 52 countries [2Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the national cholesterol education program adult treatment panel III guidelines. J Am Coll Cardiol 2004; 44(3): 720-32.
[http://dx.doi.org/10.1016/j.jacc.2004.07.001] [PMID: 15358046] ]. Extensive research indicates elevated LDL-C cholesterol is a major cause of Coronary Heart Disease (CHD). Clinical trials have shown that LDL-C-lowering therapy reduces the risk for CHD. Therefore, initiation of treatment is based on LDL-C levels and target of treatment is LDL-C [3Hong YM. Atherosclerotic cardiovascular disease beginning in childhood. Korean Circ J 2010; 40(1): 1-9.
[http://dx.doi.org/10.4070/kcj.2010.40.1.1] [PMID: 20111646] , 4Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-control study. Lancet 2004; 364(9438): 937-52.
[http://dx.doi.org/10.1016/S0140-6736(04)17018-9] [PMID: 15364185] ]. Nonetheless, in a cohort, it was observed that almost 70% of hospitalized patients, due to coronary artery disease, had LDL-C levels lower than 100 mg/dL upon admission [5Sachdeva A, Cannon CP, Deedwania PC, et al. Lipid levels in patients hospitalized with coronary artery disease: An analysis of 136,905 hospitalizations in Get With The Guidelines. American heart journal 2009; 157(e2.): 111-7.].
Undoubtedly there is a complex association between lipid metabolism, atherosclerosis and inflammation [6Ross R. Atherosclerosis: An inflammatory disease. N Engl J Med 1999; 340(2): 115-26.
[http://dx.doi.org/10.1056/NEJM199901143400207] [PMID: 9887164] ]. Insights into atherosclerosis as an inflammatory disease offer the opportunity to find new risk predictors and develop novel therapeutic strategies targeting the inflammatory component of the disease or more preferably directly targeting the culprit initiating and perpetuating the inflammatory process. Chronic tissue inflammation translates into inflammatory cytokines in the system. Cytokine secretion and activation can result in damage to the vascular endothelium priming the environment towards atherogenesis or causing existing plaque rupture, thrombosis, and even acute ischemic symptoms [7Getz GS, Reardon CA. The mutual interplay of lipid metabolism and the cells of the immune system in relation to atherosclerosis. Clin Lipidol 2014; 9(6): 657-71.
[http://dx.doi.org/10.2217/clp.14.50] [PMID: 25705263] ]. Lipids can also modulate the inflammatory response, but inflammatory signaling can significantly alter lipid metabolism in the context of atherosclerosis [8Ramji DP, Davies TS. Cytokines in atherosclerosis: Key players in all stages of disease and promising therapeutic targets. Cytokine Growth Factor Rev 2015; 26(6): 673-85.
[http://dx.doi.org/10.1016/j.cytogfr.2015.04.003] [PMID: 26005197] ]. Anti-inflammatory and immunosuppressive mechanisms inhibit atherosclerosis and may be attractive targets for disease prevention and/or treatment [9Bäck M, Hansson GK. Anti-inflammatory therapies for atherosclerosis. Nat Rev Cardiol 2015; 12(4): 199-211.
[http://dx.doi.org/10.1038/nrcardio.2015.5] [PMID: 25666404] ]. Among inflammatory mediators, raised plasma levels of C-Reactive Protein (CRP) is associated with increased risk of coronary heart diseases [10Kaptoge S, Di Angelantonio E, Lowe G, et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: An individual participant meta-analysis. Lancet 2010; 375(9709): 132-40.
[http://dx.doi.org/10.1016/S0140-6736(09)61717-7] [PMID: 20031199] ]. In addition, IL-6 and IL-1β are CRP upstream mediators associated with cardiovascular events [11Ridker PM. From c-reactive protein to interleukin-6 to interleukin-1: Moving upstream to identify novel targets for atheroprotection. Circ Res 2016; 118(1): 145-56.
[http://dx.doi.org/10.1161/CIRCRESAHA.115.306656] [PMID: 26837745] ]. IL-17A is signature cytokine of Th17 cells associated with inflammatory and autoimmune diseases [12Burkett PR, Meyer zu Horste G, Kuchroo VK. Pouring fuel on the fire: Th17 cells, the environment, and autoimmunity. J Clin Invest 2015; 125(6): 2211-9.
[http://dx.doi.org/10.1172/JCI78085] [PMID: 25961452] ], and its association with atherosclerosis is still controversial. Th17 differentiation is promoted by a set of cytokines which include TGF-β. TGF-β induces the RAR-related orphan receptor C (RORc) as the master transcription factor to direct the Th17 differentiation program in humans [13Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol 2009; 27: 485-517.
[http://dx.doi.org/10.1146/annurev.immunol.021908.132710] [PMID: 19132915] ]. To the best of our knowledge, this is the first study to measure IL-17A, IL-23 and TGF-β along with LDL-C in healthy adults. The aim of this study was to investigate any possible association between serum levels of TGF-β, IL-23, IL-17A and LDL-C in healthy people with increased levels of LDL-C.
2. MATERIALS AND METHODS
2.1. Participants
Study samples were collected from Jul. to Sep. 2011 from the “Diabetes Screening Project” conducted on a volunteer population at Tehran’s Farmanfarmaian Health Center. In addition, a few subjects were recruited from healthy workers referred to the same center for their periodical medical tests in compliance with the Occupational Medicine Rules of Iran. After gaining approval from the ethics committee of TUMS and obtaining informed consents, background information such as weight, height and age were recorded. Subjects with known disease were excluded. Serum samples of the remaining subjects were collected and stored at -20 °C and biochemical tests were conducted for 120 people.
Using autoanalyzer (Hitachi 902, Japan) Fasting Blood Glucose (FBS), total cholesterol, High- Density Lipoprotein Cholesterol (HDL-C) and triglyceride (TG) were measured. LDL-C was measured using commercial kits (Parsazmun, Iran).
After conducting biochemical tests, all subjects with FBS =>105 mg/dL were also excluded. Finally, 74 subjects (42 males and 32 females) were selected for the present study. According to the serum LDL-C levels, these subjects were divided into two groups (i.e. people with LDL-C =>130 mg/dL or LDL-C <130 mg/dL) [3Hong YM. Atherosclerotic cardiovascular disease beginning in childhood. Korean Circ J 2010; 40(1): 1-9.
[http://dx.doi.org/10.4070/kcj.2010.40.1.1] [PMID: 20111646] ].
2.2. Cytokine Assays
Serum concentrations of IL-17A, IL-23 and TGF-β were measured using commercially available enzyme-linked immunosorbent assay kits (eBioscience ELISA kits,USA) as described previously [14Roohi A, Tabrizi M, Abbasi F, et al. Serum IL-17, IL-23, and TGF-β levels in type 1 and type 2 diabetic patients and agematched healthy controls. BioMed Res Int 2014; 2014: 718946.
[http://dx.doi.org/10.1155/2014/718946] [PMID: 24995325] ]. Sensitivity of the kits was follows: IL-17A; 4 pg/ml, IL-23; 15 pg/ml and TGF-β; 60 pg/ml. In brief, Maxisorp immuno plates (Nunc, Denmark) were coated with monoclonal antibodies (mAb) specific for IL-17A, IL-23 or TGF-β. Then, serum samples and standards were added and serum cytokines were detected using biotinylated mAb specific for IL-17A, IL-23 or TGF-β followed by addition of streptavidin-horseradish peroxidase and color development. Absorbance was read at 450 nm. Using standard curves, the values were expressed in picogram per milliliter.
2.3. Statistical Analysis
All data are presented as means ± SD. To compare groups for continuous variables, Mann-Whitney test was used. To determine the association of IL-17A, IL-23 and TGF-β with other parameters, Pearson and Spearman correlation tests were conducted. In all analyses, p values <0.05 were considered significant. All statistics were done using SPSS for windows version 19.
3. RESULTS
This study was conducted to investigate any association between IL-17A, IL-23, TGF-β and LDL-C in people with LDL-C higher than normal range (130 mg/dL). Table 1 shows the demographic and clinical characteristics of the study subjects.
Statistical analysis showed a significant difference between serum IL-17A levels in subjects with LDL =>130 and those who had LDL-C<130. No significant difference was found between two groups in terms of IL-23 and TGF-β serum levels. Results are summarized in Fig. 1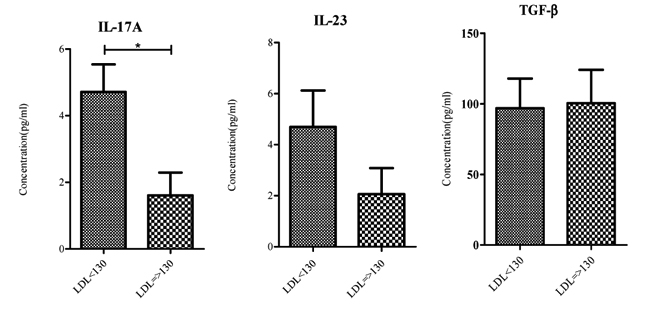 and Table 2 .
and Table 2 .
 |
Fig. (1) Serum IL-17A, IL-23 and TGF-β levels in study subjects. IL-17A levels were significantly lower in subject with LDL-C => 130 *p value < 0.05. |
Using Pearson correlation coefficients, relations between LDL-C and the three mentioned cytokines were analyzed. Results of the analysis indicated no relation between these cytokines and LDL-C while data provide support for a correlation between IL-17A levels and IL-23 levels in serum as was expected (r = 0.771).
4. DISCUSSION
During acute infections, hypertriglyceridemia can occur due to tumor necrosis factor α (TNF-α) effects on hepatic lipogenesis and lipoprotein lipase. In addition, noticeable concentrations of cholesterol are needed for proliferation of activated T cells which requires cholesterol mobilization. This relationship between metabolism and the immune system is multi-faceted in chronic diseases such as rheumatoid arthritis and atherosclerosis [15Gisterå A, Hansson GK. The immunology of atherosclerosis. Nat Rev Nephrol 2017; 13(6): 368-80.
[http://dx.doi.org/10.1038/nrneph.2017.51] [PMID: 28392564] ] in which a vicious cycle links hypercholesterolemia and inflammation. LDL-C is the leading atherogenic cholesterol carrier lipoprotein. Elevation of plasma levels of LDL-C promotes its retention in the arterial intima. Various modified forms of LDL-C, such as oxidized or aggregated LDL-C can also bind scavenger and Toll-like receptors on macrophages or be taken up via micropinocytosis or phagocytosis by macrophages [16Buono C, Anzinger JJ, Amar M, Kruth HS. Fluorescent pegylated nanoparticles demonstrate fluid-phase pinocytosis by macrophages in mouse atherosclerotic lesions. J Clin Invest 2009; 119(5): 1373-81.
[http://dx.doi.org/10.1172/JCI35548] [PMID: 19363293] ]. In addition to macrophages, lipids can activate endothelial cells with the capability to express adhesion molecules, and, of course, adhesion molecule expression is enhanced by turbulent blood flow [17Brown AJ, Teng Z, Evans PC, Gillard JH, Samady H, Bennett MR. Role of biomechanical forces in the natural history of coronary atherosclerosis. Nat Rev Cardiol 2016; 13(4): 210-20.
[http://dx.doi.org/10.1038/nrcardio.2015.203] [PMID: 26822720] ]. Upregulation of adhesion molecules and chemokine production culminate in the recruitment of other immune cells including T and B cells. Indeed, in advanced atherosclerosis, ectopic lymphoid organs are generated [18Corsiero E, Nerviani A, Bombardieri M, Pitzalis C. Ectopic lymphoid structures: Powerhouse of autoimmunity. Front Immunol 2016; 7: 430.
[http://dx.doi.org/10.3389/fimmu.2016.00430] [PMID: 27799933] ]. Considering all these events, it seems reasonable to expect an association between LDL-C levels and inflammatory mediators.
In the present study, the existence of a positive association between LDL-C and serum IL-17A level, as an inflammatory marker, was hypothesized. We measured serum levels of IL-17A, IL-23 and TGF-β in healthy adults who demonstrated LDL-C levels higher than 130 mg/dL. Our aim was to find an association between LDL-C and IL-17A as an inflammatory cytokine in atheroma development before any disease observation. The serum levels of IL-23 and TGF-β, as effective cytokines in the development of Th17 cells, were also determined. The results indicated that the levels of IL-17A demonstrated reverse association with LDL-C.
Physiologically, development of atherosclerotic plaques is a lengthy and complicated process in which the immune system assumes a Janus-faced identity [19Hansson GK, Libby P. The immune response in atherosclerosis: A double-edged sword. Nat Rev Immunol 2006; 6(7): 508-19.
[http://dx.doi.org/10.1038/nri1882] [PMID: 16778830] ]. As mentioned above, it seems that an interplay between modified LDL-C or associated LDL-C molecules and the innate immune system triggers plaque formation followed by acquired immune system activation. Macrophages, as key cellular players in plaque formation, gain various phenotypes and characteristics affected by lipids, cytokines, irons and senesced cells [20Colin S, Chinetti-Gbaguidi G, Staels B. Macrophage phenotypes in atherosclerosis. Immunol Rev 2014; 262(1): 153-66.
[http://dx.doi.org/10.1111/imr.12218] [PMID: 25319333] ]. These cells modulate atheroma progression in part via secretion of anti-atherosclerotic (TGF-β, IL-10) and/or pro-atherosclerotic mediators (IL-6, TNF-α) [21Tabas I, Bornfeldt KE. Macrophage phenotype and function in different stages of atherosclerosis. Circ Res 2016; 118(4): 653-67.
[http://dx.doi.org/10.1161/CIRCRESAHA.115.306256] [PMID: 26892964] ].
In addition to the innate immune system, there are many reports regarding opposing roles played by different T cell subsets in atherosclerosis. Th1 cells produce interferon γ (IFN-γ). This cytokine induces foam cell formation, macrophage activation and prohibits smooth muscle cell proliferation and differentiation which results in plaque destabilization [22Hansson GK, Hellstrand M, Rymo L, Rubbia L, Gabbiani G. Interferon gamma inhibits both proliferation and expression of differentiation-specific alpha-smooth muscle actin in arterial smooth muscle cells. J Exp Med 1989; 170(5): 1595-608.
[http://dx.doi.org/10.1084/jem.170.5.1595] [PMID: 2509626] ]. As it is expected, natural regulatory T cells (TReg) show atheroprotective effects via TGF-β and IL-10 production [23George J. Mechanisms of disease: The evolving role of regulatory T cells in atherosclerosis. Nat Clin Pract Cardiovasc Med 2008; 5(9): 531-40.
[http://dx.doi.org/10.1038/ncpcardio1279] [PMID: 18607396] ]. Role of Th17 and its signature cytokine, IL-17A, in atherosclerosis is controversial [24Ryu H, Chung Y. Regulation of IL-17 in atherosclerosis and related autoimmunity. Cytokine 2015; 74(2): 219-27.
[http://dx.doi.org/10.1016/j.cyto.2015.03.009] [PMID: 25890878] , 25Taleb S, Tedgui A. IL-17 in atherosclerosis: The good and the bad. Cardiovasc Res 2018; 114(1): 7-9.
[http://dx.doi.org/10.1093/cvr/cvx225] [PMID: 29228116] ]. In a review of recent literature, Yu et al. concluded that IL-17A seems to have atherogenic properties [26Yu XH, Jiang N, Zheng XL, Cayabyab FS, Tang ZB, Tang CK. Interleukin-17A in lipid metabolism and atherosclerosis. Clin Chim Acta 2014; 431: 33-9.
[http://dx.doi.org/10.1016/j.cca.2014.01.012] [PMID: 24508995] ]. In a research conducted on apolipoprotein E-deficient mice, deficiency of IL-17A resulted in accelerated formation of unstable atherosclerotic plaques [27Danzaki K, Matsui Y, Ikesue M, et al. Interleukin-17A deficiency accelerates unstable atherosclerotic plaque formation in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol 2012; 32(2): 273-80.
[http://dx.doi.org/10.1161/ATVBAHA.111.229997] [PMID: 22116098] ]. Gistera et al. observed that TGF-β can stabilize plaques via an IL-17A-dependent pathway [28Gistera A, Robertson AK, Andersson J, et al. Transforming growth factor-beta signaling in T cells promotes stabilization of atherosclerotic plaques through an interleukin-17-dependent pathway. Science translational medicine 2013; 5: 196-ra00.] while it is reported that blockade of IL-17A resulted in decreased lesion size and stabilization [29Erbel C, Akhavanpoor M, Okuyucu D, et al. IL-17A influences essential functions of the monocyte/macrophage lineage and is involved in advanced murine and human atherosclerosis. J Immunol 2014; 193(9): 4344-55.
[http://dx.doi.org/10.4049/jimmunol.1400181] [PMID: 25261478] ]. Thus, IL-17A may be serving a dual function depending on the mechanism that is controlling it. If IL-17A is activated by TGF-β, it stabilizes plaques. On the other hand, IL-17A suppression by a yet unknown mechanism, leads to lesion size reduction and stabilization.
In addition to the immune system, there are elements which exert counteracting effects on inflammation promotion during atheroma formation. Interestingly, cholesterol itself plays contradictory roles. Accumulation of cholesterol inside macrophages leads to increased activation of Toll-like receptors and inflammasome formation and also production of more monocytes and neutrophils. On the other hand, cholesterol can activate a heterodimeric transcription factor, liver X receptor (LXR)–retinoid X receptor (RXR), with anti-inflammatory effects [30Tall AR, Yvan-Charvet L. Cholesterol, inflammation and innate immunity. Nat Rev Immunol 2015; 15(2): 104-16.
[http://dx.doi.org/10.1038/nri3793] [PMID: 25614320] ]. It is not counterintuitive to observe protective mechanisms being activated in response to assaults on normal human physiology (pathology).
Surendar et al. have unexpectedly found a decrease of IL-17A levels in subjects with metabolic syndrome (MS) abnormalities [31Surendar J, Aravindhan V, Rao MM, Ganesan A, Mohan V. Decreased serum interleukin-17 and increased transforming growth factor-β levels in subjects with metabolic syndrome (Chennai Urban Rural Epidemiology Study-95). Metabolism 2011; 60(4): 586-90.
[http://dx.doi.org/10.1016/j.metabol.2010.06.003] [PMID: 20667562] ]. Metabolic syndrome is a group of CHD and diabetes risk factors including abnormal levels of cholesterol and TG. In MS inflammatory pathways are also activated [32Catrysse L, van Loo G. Inflammation and the metabolic syndrome: The tissue-specific functions of NF-κB. Trends Cell Biol 2017; 27(6): 417-29.
[http://dx.doi.org/10.1016/j.tcb.2017.01.006] [PMID: 28237661] ].In Surendar study, subjects with MS had TG levels which were significantly higher than the control group while there was no significant difference between LDL-C levels in their case and control groups [31Surendar J, Aravindhan V, Rao MM, Ganesan A, Mohan V. Decreased serum interleukin-17 and increased transforming growth factor-β levels in subjects with metabolic syndrome (Chennai Urban Rural Epidemiology Study-95). Metabolism 2011; 60(4): 586-90.
[http://dx.doi.org/10.1016/j.metabol.2010.06.003] [PMID: 20667562] ]. It seems that our findings regarding IL17-A are in line with the study conducted by Surendar et al. In the present study, the TG level along with LDL-C levels showed significant differences between the two study groups. It was significantly higher in subjects with LDL-C=>130(p <0.001). It would be intriguing to study any possible relationship between TG and IL-17A. Since IL-23 is a major Th17 cell inducer and there is a positive correlation between IL-23 and IL-17A levels, it seems reasonable to observe a similar positive correlation between IL-23 and IL-17A levels in plasma. In the present study, we observed rise in IL-23 levels corresponded to a rise in IL-17A levels in study subjects sera.
In the present study, TGF-β levels were measured because TGF-β is a cytokine required for Th17A development [13Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol 2009; 27: 485-517.
[http://dx.doi.org/10.1146/annurev.immunol.021908.132710] [PMID: 19132915] ], but no significant difference between the two study groups was observed. TGF-β affects different cell types involved in atheroma development including macrophages, endothelial cells and vascular smooth muscle cells. The major effect of TGF-β on macrophages is anti-atherogenic. However, function of this cytokine in atherosclerosis progression is not clear due to its roles in fibrosis promotion and inhibition of endothelial regeneration [33Singh NN, Ramji DP. The role of transforming growth factor-beta in atherosclerosis. Cytokine Growth Factor Rev 2006; 17(6): 487-99.
[http://dx.doi.org/10.1016/j.cytogfr.2006.09.002] [PMID: 17056295] ]. Recently conducted research has revealed a mutual interplay between TGF-β and lipids. The TGF-β signaling pathway has been shown to be dysregulated by cholesterol in a variety of cell lines [34Chen CL, Huang SS, Huang JS. Cholesterol modulates cellular TGF-beta responsiveness by altering TGF-beta binding to TGF-beta receptors. J Cell Physiol 2008; 215(1): 223-33.
[http://dx.doi.org/10.1002/jcp.21303] [PMID: 17972267] ]. In atherosclerotic mice, researchers have found positive correlation between cholesterol and TGF-β levels in plasma. It was speculated that elevated levels of TGF-β may neutralize pro-inflammatory effects of hypercholesterolemia [35Zhou X, Johnston TP, Johansson D, et al. Hypercholesterolemia leads to elevated TGF-beta1 activity and T helper 3-dependent autoimmune responses in atherosclerotic mice. Atherosclerosis 2009; 204(2): 381-7.
[http://dx.doi.org/10.1016/j.atherosclerosis.2008.10.017] [PMID: 19054515] ]. In patients with advanced atherosclerosis, reduced plasma levels of this cytokine have been reported [36Grainger DJ, Kemp PR, Metcalfe JC, et al. The serum concentration of active transforming growth factor-beta is severely depressed in advanced atherosclerosis. Nat Med 1995; 1(1): 74-9.
[http://dx.doi.org/10.1038/nm0195-74] [PMID: 7584958] ] meaning that different concentrations of this cytokine during atherosclerosis progression may be associated with disease stage [37Toma I, McCaffrey TA. Transforming growth factor-β and atherosclerosis: Interwoven atherogenic and atheroprotective aspects. Cell Tissue Res 2012; 347(1): 155-75.
[http://dx.doi.org/10.1007/s00441-011-1189-3] [PMID: 21626289] ].
CONCLUSION
This is a cross-sectional study on healthy adults with elevated levels of LDL-C who may or may not develop atherosclerotic plaques in the future. No causal relation may be found between measured cytokines and atherosclerosis incidence but any predictable associations can allow planning of interventions to prevent atherosclerotic diseases. Recently, the application of canakinumab, a monoclonal antibody against IL-1β, effectively reduced the rate of cardiovascular events [38Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017; 377(12): 1119-31.
[http://dx.doi.org/10.1056/NEJMoa1707914] [PMID: 28845751] ]. Meanwhile, further studies of similar nature may allow scientists to shed more light on the lengthy and complicated mechanism of atheroma formation and discovery of related inflammatory/anti-inflammatory predictors.
LIST OF ABBREVIATIONS
| LDL-C | = Low- density lipoprotein cholesterol |
| IL-17A | = Interleukin 17A |
| IL-23 | = Interleukin 23 |
| TGF- β | = Transforming growth factor β |
| Th17 | = T helper 17 |
| ELISA | = Enzyme-linked immunosorbent assay |
| MI | = Myocardial infarction |
| CHD | = Coronary heart disease |
| CRP | = C-reactive protein |
| RORc | = RAR-related orphan receptor C |
| FBS | = Fasting blood glucose |
| HDL-C | = High- density lipoprotein cholesterol |
| TG | = Triglyceride |
| mAb | = Monoclonal antibodies |
| TNF-α | = Tumor necrosis factor α |
| IFN γ | = Interferon γ |
| Treg | = Regulatory T cells |
| LXR | = Liver X receptor |
| RXR | = Retinoid X receptor |
| MS | = Metabolic syndrome |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the ethics committee of Tehran University of Medical Sciences. Written informed consents were obtained from all participants.
HUMAN AND ANIMAL RIGHTS
No animal was used for this study.
CONSENT FOR PUBLICATION
The publisher has the authors' permission to publish this study.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
This study was financially supported by Grant no. 88-03-87-9424 from the Deputy of Research of Tehran University of Medical Sciences to Behrouz Nikbin.
REFERENCES
| [1] | Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA 2001; 285(19): 2486-97. [http://dx.doi.org/10.1001/jama.285.19.2486] [PMID: 11368702] |
| [2] | Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the national cholesterol education program adult treatment panel III guidelines. J Am Coll Cardiol 2004; 44(3): 720-32. [http://dx.doi.org/10.1016/j.jacc.2004.07.001] [PMID: 15358046] |
| [3] | Hong YM. Atherosclerotic cardiovascular disease beginning in childhood. Korean Circ J 2010; 40(1): 1-9. [http://dx.doi.org/10.4070/kcj.2010.40.1.1] [PMID: 20111646] |
| [4] | Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-control study. Lancet 2004; 364(9438): 937-52. [http://dx.doi.org/10.1016/S0140-6736(04)17018-9] [PMID: 15364185] |
| [5] | Sachdeva A, Cannon CP, Deedwania PC, et al. Lipid levels in patients hospitalized with coronary artery disease: An analysis of 136,905 hospitalizations in Get With The Guidelines. American heart journal 2009; 157(e2.): 111-7. |
| [6] | Ross R. Atherosclerosis: An inflammatory disease. N Engl J Med 1999; 340(2): 115-26. [http://dx.doi.org/10.1056/NEJM199901143400207] [PMID: 9887164] |
| [7] | Getz GS, Reardon CA. The mutual interplay of lipid metabolism and the cells of the immune system in relation to atherosclerosis. Clin Lipidol 2014; 9(6): 657-71. [http://dx.doi.org/10.2217/clp.14.50] [PMID: 25705263] |
| [8] | Ramji DP, Davies TS. Cytokines in atherosclerosis: Key players in all stages of disease and promising therapeutic targets. Cytokine Growth Factor Rev 2015; 26(6): 673-85. [http://dx.doi.org/10.1016/j.cytogfr.2015.04.003] [PMID: 26005197] |
| [9] | Bäck M, Hansson GK. Anti-inflammatory therapies for atherosclerosis. Nat Rev Cardiol 2015; 12(4): 199-211. [http://dx.doi.org/10.1038/nrcardio.2015.5] [PMID: 25666404] |
| [10] | Kaptoge S, Di Angelantonio E, Lowe G, et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: An individual participant meta-analysis. Lancet 2010; 375(9709): 132-40. [http://dx.doi.org/10.1016/S0140-6736(09)61717-7] [PMID: 20031199] |
| [11] | Ridker PM. From c-reactive protein to interleukin-6 to interleukin-1: Moving upstream to identify novel targets for atheroprotection. Circ Res 2016; 118(1): 145-56. [http://dx.doi.org/10.1161/CIRCRESAHA.115.306656] [PMID: 26837745] |
| [12] | Burkett PR, Meyer zu Horste G, Kuchroo VK. Pouring fuel on the fire: Th17 cells, the environment, and autoimmunity. J Clin Invest 2015; 125(6): 2211-9. [http://dx.doi.org/10.1172/JCI78085] [PMID: 25961452] |
| [13] | Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol 2009; 27: 485-517. [http://dx.doi.org/10.1146/annurev.immunol.021908.132710] [PMID: 19132915] |
| [14] | Roohi A, Tabrizi M, Abbasi F, et al. Serum IL-17, IL-23, and TGF-β levels in type 1 and type 2 diabetic patients and agematched healthy controls. BioMed Res Int 2014; 2014: 718946. [http://dx.doi.org/10.1155/2014/718946] [PMID: 24995325] |
| [15] | Gisterå A, Hansson GK. The immunology of atherosclerosis. Nat Rev Nephrol 2017; 13(6): 368-80. [http://dx.doi.org/10.1038/nrneph.2017.51] [PMID: 28392564] |
| [16] | Buono C, Anzinger JJ, Amar M, Kruth HS. Fluorescent pegylated nanoparticles demonstrate fluid-phase pinocytosis by macrophages in mouse atherosclerotic lesions. J Clin Invest 2009; 119(5): 1373-81. [http://dx.doi.org/10.1172/JCI35548] [PMID: 19363293] |
| [17] | Brown AJ, Teng Z, Evans PC, Gillard JH, Samady H, Bennett MR. Role of biomechanical forces in the natural history of coronary atherosclerosis. Nat Rev Cardiol 2016; 13(4): 210-20. [http://dx.doi.org/10.1038/nrcardio.2015.203] [PMID: 26822720] |
| [18] | Corsiero E, Nerviani A, Bombardieri M, Pitzalis C. Ectopic lymphoid structures: Powerhouse of autoimmunity. Front Immunol 2016; 7: 430. [http://dx.doi.org/10.3389/fimmu.2016.00430] [PMID: 27799933] |
| [19] | Hansson GK, Libby P. The immune response in atherosclerosis: A double-edged sword. Nat Rev Immunol 2006; 6(7): 508-19. [http://dx.doi.org/10.1038/nri1882] [PMID: 16778830] |
| [20] | Colin S, Chinetti-Gbaguidi G, Staels B. Macrophage phenotypes in atherosclerosis. Immunol Rev 2014; 262(1): 153-66. [http://dx.doi.org/10.1111/imr.12218] [PMID: 25319333] |
| [21] | Tabas I, Bornfeldt KE. Macrophage phenotype and function in different stages of atherosclerosis. Circ Res 2016; 118(4): 653-67. [http://dx.doi.org/10.1161/CIRCRESAHA.115.306256] [PMID: 26892964] |
| [22] | Hansson GK, Hellstrand M, Rymo L, Rubbia L, Gabbiani G. Interferon gamma inhibits both proliferation and expression of differentiation-specific alpha-smooth muscle actin in arterial smooth muscle cells. J Exp Med 1989; 170(5): 1595-608. [http://dx.doi.org/10.1084/jem.170.5.1595] [PMID: 2509626] |
| [23] | George J. Mechanisms of disease: The evolving role of regulatory T cells in atherosclerosis. Nat Clin Pract Cardiovasc Med 2008; 5(9): 531-40. [http://dx.doi.org/10.1038/ncpcardio1279] [PMID: 18607396] |
| [24] | Ryu H, Chung Y. Regulation of IL-17 in atherosclerosis and related autoimmunity. Cytokine 2015; 74(2): 219-27. [http://dx.doi.org/10.1016/j.cyto.2015.03.009] [PMID: 25890878] |
| [25] | Taleb S, Tedgui A. IL-17 in atherosclerosis: The good and the bad. Cardiovasc Res 2018; 114(1): 7-9. [http://dx.doi.org/10.1093/cvr/cvx225] [PMID: 29228116] |
| [26] | Yu XH, Jiang N, Zheng XL, Cayabyab FS, Tang ZB, Tang CK. Interleukin-17A in lipid metabolism and atherosclerosis. Clin Chim Acta 2014; 431: 33-9. [http://dx.doi.org/10.1016/j.cca.2014.01.012] [PMID: 24508995] |
| [27] | Danzaki K, Matsui Y, Ikesue M, et al. Interleukin-17A deficiency accelerates unstable atherosclerotic plaque formation in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol 2012; 32(2): 273-80. [http://dx.doi.org/10.1161/ATVBAHA.111.229997] [PMID: 22116098] |
| [28] | Gistera A, Robertson AK, Andersson J, et al. Transforming growth factor-beta signaling in T cells promotes stabilization of atherosclerotic plaques through an interleukin-17-dependent pathway. Science translational medicine 2013; 5: 196-ra00. |
| [29] | Erbel C, Akhavanpoor M, Okuyucu D, et al. IL-17A influences essential functions of the monocyte/macrophage lineage and is involved in advanced murine and human atherosclerosis. J Immunol 2014; 193(9): 4344-55. [http://dx.doi.org/10.4049/jimmunol.1400181] [PMID: 25261478] |
| [30] | Tall AR, Yvan-Charvet L. Cholesterol, inflammation and innate immunity. Nat Rev Immunol 2015; 15(2): 104-16. [http://dx.doi.org/10.1038/nri3793] [PMID: 25614320] |
| [31] | Surendar J, Aravindhan V, Rao MM, Ganesan A, Mohan V. Decreased serum interleukin-17 and increased transforming growth factor-β levels in subjects with metabolic syndrome (Chennai Urban Rural Epidemiology Study-95). Metabolism 2011; 60(4): 586-90. [http://dx.doi.org/10.1016/j.metabol.2010.06.003] [PMID: 20667562] |
| [32] | Catrysse L, van Loo G. Inflammation and the metabolic syndrome: The tissue-specific functions of NF-κB. Trends Cell Biol 2017; 27(6): 417-29. [http://dx.doi.org/10.1016/j.tcb.2017.01.006] [PMID: 28237661] |
| [33] | Singh NN, Ramji DP. The role of transforming growth factor-beta in atherosclerosis. Cytokine Growth Factor Rev 2006; 17(6): 487-99. [http://dx.doi.org/10.1016/j.cytogfr.2006.09.002] [PMID: 17056295] |
| [34] | Chen CL, Huang SS, Huang JS. Cholesterol modulates cellular TGF-beta responsiveness by altering TGF-beta binding to TGF-beta receptors. J Cell Physiol 2008; 215(1): 223-33. [http://dx.doi.org/10.1002/jcp.21303] [PMID: 17972267] |
| [35] | Zhou X, Johnston TP, Johansson D, et al. Hypercholesterolemia leads to elevated TGF-beta1 activity and T helper 3-dependent autoimmune responses in atherosclerotic mice. Atherosclerosis 2009; 204(2): 381-7. [http://dx.doi.org/10.1016/j.atherosclerosis.2008.10.017] [PMID: 19054515] |
| [36] | Grainger DJ, Kemp PR, Metcalfe JC, et al. The serum concentration of active transforming growth factor-beta is severely depressed in advanced atherosclerosis. Nat Med 1995; 1(1): 74-9. [http://dx.doi.org/10.1038/nm0195-74] [PMID: 7584958] |
| [37] | Toma I, McCaffrey TA. Transforming growth factor-β and atherosclerosis: Interwoven atherogenic and atheroprotective aspects. Cell Tissue Res 2012; 347(1): 155-75. [http://dx.doi.org/10.1007/s00441-011-1189-3] [PMID: 21626289] |
| [38] | Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017; 377(12): 1119-31. [http://dx.doi.org/10.1056/NEJMoa1707914] [PMID: 28845751] |