- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

Current Chemical Genomics and Translational Medicine
(Discontinued)
ISSN: 2213-9885 ― Volume 12, 2018
A Novel Fluorogenic Coumarin Substrate for Monitoring Acid Phosphatase Activity at Low pH Environment
Desuo Yang1, Zongxiao Li1, Yubo Allan Diwu3, Hanzhuo Fu2, Jinfang Liao3, Chunmei Wei3, Zhenjun Diwu*, 3
Abstract
This article described the synthesis and application of 6-chloro-8-fluoro-4-methylumbelliferone phosphate (CF-MUP) in analyzing acid phosphatase activity. Compared to the existing MUP, the new coumarin phosphate, CF-MUP, demonstrateed much higher sensitivity and was more robust for detecting the activity of acid phosphatase than the classic substrate 4-methylumbelliferone phosphate (MUP). The product of enzyme reaction, 6-chloro-8-fluoro-4-methylumbelliferone (CF-MU) possesses strong fluorescence at ~450 nm with low pKa (4.7), high fluorescence quantum yield and pH independence in the physiological pH range. This new fluorescence dye, CF-MU, is a convenient tool for assays with buffer pH between 4.5 and 8.
Article Information
Identifiers and Pagination:
Year: 2008Volume: 2
First Page: 48
Last Page: 50
Publisher Id: CCGTM-2-48
DOI: 10.2174/1875397300802010048
Article History:
Received Date: 1/9/2008Revision Received Date: 17/10/2008
Acceptance Date: 20/10/2008
Electronic publication date: 14/11/2008
Collection year: 2008
open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
* Address correspondence to this author at the ABD Bioquest, Inc., 923 Thompson Place, Sunnyvale, CA 94085, USA; E-mail: jack@abdbioquest.com
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 1-9-2008 |
Original Manuscript | A Novel Fluorogenic Coumarin Substrate for Monitoring Acid Phosphatase Activity at Low pH Environment | |
INTRODUCTION
Acid phosphatases are family of enzymes ubiquitous in nature and have been found in many tissues in animals and plants. The function of acid phosphatases is catalyzing the hydrolysis of orthophosphate monoesters under acidic condition that regulates a variety of cellular functions. Human prostatic acid phosphatase was used as a surrogate biomarker for mornitoring prostate cancer before the detection of prostate-specific antigen (PSA) became available [1Bodansky O. Acid phosphatase Adv Clin Chem 1972; 15: 43-147.-8Taira A, Merrick G, Wallner K, Dattoli M. Reviving the acid phosphatase test for prostate cancer Oncology (Williston Park) 2007; 21: 1003-.]. Acid phosphatase has also been reportedly associated with Gaucher's disease in which patients exhibit unique peaks in their electrophoresed sera [9Tyson MC, Grossman WI, Tuchman LR. Gaucher's disease (with elevated serum acid phosphatase level) masquerading as cirrhosis of the liver Am J Med 1964; 37: 156-8.-11Robinson DB, Glew RH. Acid phosphatase in Gaucher's disease Clin Chem 1980; 26: 371-82.]. The acid phosphatase catalyzes the following reaction at an optimal pH, usually under pH 7:

Clinically, the measurement of acid phosphatase activity has been used for diagnosing cancers and for monitoring cell viability. Both para-nitrophenol phosphate (pNPP) and 4-methylumbelliferyl phosphate (MUP) have been widely used for measuring acid phosphatase activity [12Chambers JP, Aquino L, Glew RH, Lee RE, McCafferty LR. Determination of serum acid phosphatase in Gaucher's disease using 4-methylumbelliferyl phosphate Clin Chim Acta 1977; 80: 67-77.-15Miyayama H, Solomon R, Sasaki M, Lin CW, Fishman WH. Demonstration of lysosomal and extralysosomal sites for acid phosphatase in mouse kidney tubule cells with p-nitrophenylphosphate lead-salt technique J Histochem Cytochem 1975; 23: 439-51.]. Although MUP is more sensitive for detecting acid phosphatase activity than pNPP, its sensitivity is still quite limited [12Chambers JP, Aquino L, Glew RH, Lee RE, McCafferty LR. Determination of serum acid phosphatase in Gaucher's disease using 4-methylumbelliferyl phosphate Clin Chim Acta 1977; 80: 67-77., 13Omene JA, Glew RH, Baig HA, Robinson DB, Brock W, Chambers JP. Determination of serum acid and alkaline phosphatase using 4-methylumbelliferyl phosphate Afr J Med Med Sci 1981; 10: 9-18.]. The detection of fluorescence product, MU (pKa = ~8.0) requires an optimal pH greater than 8.0 for its maximum sensitivity while the enzyme reaction of acid phosphatase is usually performed at an optimal pH under 7.0. Thus, the buffer pH has to be raised to 8.5 to 10 after the enzyme reaction by an addition of the stop solution with high pH in order to detect acid phosphatase activity with MUP. This two-step procedure is not convenient for automated assay environments such as clinical settings or high throughput screening labs.
This requirement of an additional step to increase in buffer pH after enzyme reaction with MUP substrate is due to the high pKa of enzymatic reaction product, MU (a 7-hydroxycoumarin analog). This fluorescent dye is typically not fully deprotonated (and therefore not maximally fluorescent) unless it is present in an environment having a pH of 9 or higher. Therefore, the detection sensitivity of assays using 7-hydroxycoumarin-based enzyme substrates such as MUP suffers at lower pH. On the other hand, the rate of enzyme reaction for acid phosphatases is very limited at neutral or higher pH. In addition to acid phosphatases, a numbers of lipid hydrolases and glycosidases such as beta-glucosidase, alpha-glucosidase and alpha-galactosidase also have optimal pH between 4 and 6. Therefore the glycoside derivatives of CF-MU should be useful for detecting the enzyme activity of these lipid hydrolases and glycosidases in a continuous assay format without a need to raise pH after the enzyme reaction.
MATERIALS AND METHODS
CF-MU and CF-MUP are commercially available from ABD Bioquest and was synthesized as shown in Scheme 1. CF-MUP is readily soluble in water and all the aqueous buffers while CF-MU is readily soluble in DMSO. Wheat germ acid phosphatase (5 U/mg) was purchased from Calzyme. All the other reagents are from Sigma Chemical Company.
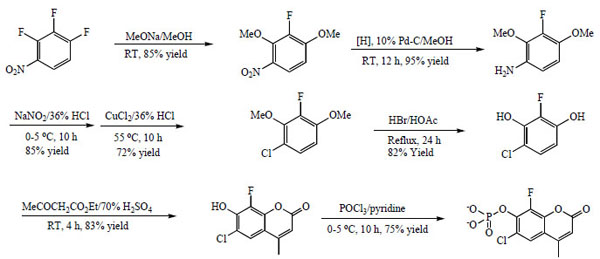 |
Scheme 1 The synthesis of CF-MUP. |
The pH Titration of Coumarins
CF-MU was first dissolved in a series of buffers that were each calibrated using a pH meter. Acetate buffers were typically used in the range of pH 4-6, and phosphate buffers in the pH range 6-8. Absorption measurements were made using solutions that were approximately 5 µM in concentration, and fluorescence measurements were made using solutions that were approximately 1 µM in concentration. The absorption or emission data was then plotted versus pH to determine pKa value using equation: pH = pKa + c[log(F-Fmin)/(Fmax-F)] [16Whitaker JE, Haugland RP, Prendergast FG. Spectral and photophysical studies of benzo[c]xanthene dyes: dual emission pH sensors Anal Biochem 1991; 194: 330-44.].
Detection of Acid Phosphatase Activity with CF-MUP
The utility of CF-MUP as a substrate for acid phosphatase was compared with MUP. For the accurate comparison, the concentrations of the two substrates (initially approximately 1 mM) were matched by normalizing the absorbance of each substrate solution at 319 nm (pH 10) to a value of 0.52 (assuming the extinction coefficient of each substrate was approximately equivalent). The matched samples were then diluted 1:10 into enzyme buffer (10 units/mL), and resulted solution was incubated at room temperature for 30 minutes at pH 5.5. The resulting fluorescence signal was recorded using excitation at 360 nm and emission at 450 nm.
RESULTS AND DISCUSSION
CF-MUP has good water solubility and weak fluorescence. It is readily converted to highly fluorescent CF-MU upon enzymatic hydrolysis by acid phosphatases. CF-MU exhibits much stronger fluorescence in aqueous solutions at low pH (Fig. 1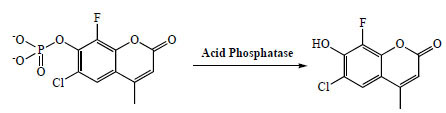 ), making CF-MUP a sensitive probe for monitoring the activity of acid phosphatase.
), making CF-MUP a sensitive probe for monitoring the activity of acid phosphatase.
 |
Fig. (1) Assay principle of CF-MUP for detecting acid phosphatase activity. |
The CF-MUP substrate overcomes the problems of non-continuous assay mode and high assay background since its reaction product, CF-MU, has a pKa of ~4.8 (Fig. 2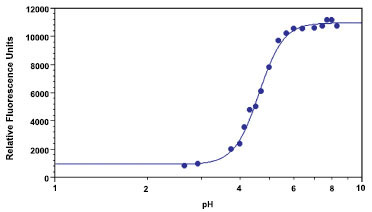 ) with the absorption peak at ~360 nm and emission peak at 450 nm, that are available in many detection instruments including microplate readers, fluorescence microscopes and flow cytometers. We found that CF-MUP is about 100 times more sensitive than MUP for the detection of acid phosphatase at pH 5.5 (Fig. 3
) with the absorption peak at ~360 nm and emission peak at 450 nm, that are available in many detection instruments including microplate readers, fluorescence microscopes and flow cytometers. We found that CF-MUP is about 100 times more sensitive than MUP for the detection of acid phosphatase at pH 5.5 (Fig. 3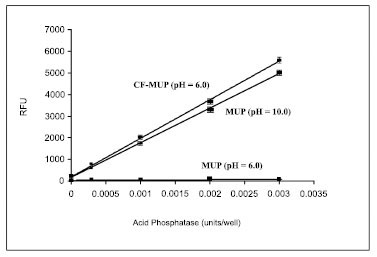 ). This higher sensitivity is resulted from both the higher turn-over rate of CF-MUP by acid phosphatase and lower pKa of CF-MU. The direct comparison of initial hydrolysis CF-MUP and MUP by acid phosphatase at pH 5.5 followed by raising pH to 10 indicated that CF-MUP is still slightly more sensitive than MUP for acid phosphatase detection (see Fig. 3
). This higher sensitivity is resulted from both the higher turn-over rate of CF-MUP by acid phosphatase and lower pKa of CF-MU. The direct comparison of initial hydrolysis CF-MUP and MUP by acid phosphatase at pH 5.5 followed by raising pH to 10 indicated that CF-MUP is still slightly more sensitive than MUP for acid phosphatase detection (see Fig. 3 ).
).
Because the enzyme reaction product of CF-MUP does not require addition of base to the reaction mixture prior to measuring the fluorescence, it can be used for the continuous assay of acid phosphatases with a wide range of pH. As seen in Fig. (2 ), CF-MU reaches its maximal fluorescence at pH 6.0, a slightly lower pKa derivative of CF-MU might further enhance acid phosphatase detection sensitivity. In addition, this CF-MU fluorophore can also be used for labeling the substrates to develop continuous assays for lipid hydrolases and glycosidases such as beta-glucosidase, alpha-glucosidase and alpha-galactosidase that have optimal pH between 4 and 6.
), CF-MU reaches its maximal fluorescence at pH 6.0, a slightly lower pKa derivative of CF-MU might further enhance acid phosphatase detection sensitivity. In addition, this CF-MU fluorophore can also be used for labeling the substrates to develop continuous assays for lipid hydrolases and glycosidases such as beta-glucosidase, alpha-glucosidase and alpha-galactosidase that have optimal pH between 4 and 6.
CONCLUSION
In conclusion, this new substrate CF-MUP demonstrated its advantage over the classic MUP for detecting the activity of acid phosphatase. The enzyme reaction product, CF-MU, possesses substantially lower pKa than MU and can be directly detected in the low pH assay condition that is usually between 4.5 and 7. This property is particularly useful for studying enzyme activity in the kinetic assay mode in low pH condition, such as acid phosphatases, lipid hydrolases and glycosidases. The excitation at 360 nm and emission at 460 nm of this new dye enables the enzyme assays compatible with the existing fluorescence detection instruments that have been set up for the MUP assays.
ACKNOWLEDGEMENT
The authors acknowledge the Key Laboratory Construction Support from the Education Office of Shaanxi Province to DY (Project No. 05JS43).




