- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

Open Chemistry Journal
(Discontinued)
ISSN: 1874-8422 ― Volume 8, 2021
Crystal Structure of the Monoprotonated Tripodal Ligand Tris(2,2’- bipyrid-6-yl) Methanol
Cuhananthan W. Sathiyajith*, James C. Knight, Benson M. Kariuki, Peter G. Edwards, Angelo J. Amoroso*
Abstract
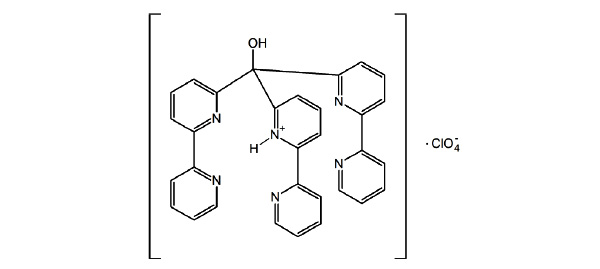
The tripodal bipyridine-based ligand tris(2,2’-bipyrid-6-yl) methanol (L 1 ) was shown in our previous work to have a strong steric preference for trigonal prismatic co - ordination environments with a series of transition metals. We now report the crystal structure of the ligand framework, isolated in its monoprotonated form with a perchlorate counterion. The structure was solved in a monoclinic space group C2/c with cell parameters, a = 21.5885(3), b = 11.7485(3), c = 24.6939(6) Å, α = 90°, β = 110.790(1) °, γ = 90°, volume = 5855.4(2) Å3 , Z = 8. The 1H NMR of the protonated ligand is similar to the parent ligand and showed the compound retained its 3-fold symmetry and all bipyridine groups were equivalent.
We report the crystal structure of the protonated form a tripodal bipyridine-based ligand framework with a strong steric preference for trigonal prismatic co-ordination environments.
Article Information
Identifiers and Pagination:
Year: 2014Volume: 1
First Page: 15
Last Page: 20
Publisher Id: CHEM-1-15
DOI: 10.2174/1874842201401010015
Article History:
Received Date: 24/07/2014Revision Received Date: 05/09/2014
Acceptance Date: 15/09/2014
Electronic publication date: 27/11/2014
Collection year: 2014
open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
* Address correspondence to this author at the School of Chemistry Cardiff University Main Building, Park Place, Cardiff CF10 3AT UK; Tel: +44 (0)29 208 74077; Fax: +44 (0)29 208 74030; E-mails: AmorosoAJ@cardiff.ac.uk and sjithcw@gmail.com
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 24-07-2014 |
Original Manuscript | Crystal Structure of the Monoprotonated Tripodal Ligand Tris(2,2’- bipyrid-6-yl) Methanol | |
INTRODUCTION
Self assembly is common place in nature [1Whitesides, GM; Boncheva, M Beyond molecules: Self-assembly of mesoscopic and macroscopic components Proc. Natl. Acad. Sci.
U S A, 2002, 99, 4769-4774.
[http://dx.doi.org/10.1073/pnas.082065899] ] and coordination chemists have provided a new dimension to this natural phenomenon through the driven self assembly of various metal ions with multidentate ligands. For six co-ordinate complexes, the octahedral geometry is overwhelming more common than the trigonal prismatic arrangement. However such arrangements have received intermittent interest in comparison to their octahedral counterparts and recently, they have been investigated for their usefulness to generate molecular assemblies ranging from the macroscale [2Hu, WJ; Liu, LQ; Ma, ML; Zhao, XL; Liu, YA; Mi, XQ; Jiang, B; Wen, K A Trigonal Prismatic Ligand in the Metal-Mediated Self-Assembly of One- and Two-Dimensional Metallosupramolecular Polymers Inorg. Chem, 2013, 52, 9309-9319.] to nano scale [3Company, A; Roques, N; Güell, M; Mugnaini, V; Gómez, L; Imaz, I; Datcu, A; Solà, M; Luis, JM; Veciana, J; Ribas, X; Costas, M Nanosized trigonal prismatic and antiprismatic CuII coordination cages based on tricarboxylate linkers Dalton. Trans, 2008, 37(13), 1679-1682.].
In order to rationalize why specific six-coordinate complexes are biased towards trigonal prismatic character, we generally consider repulsive ligand-ligand interactions, sterochemical predispositions of a ligand as well as the stereo-electronic preferences of metal ions [4Gillum, WO; Huffman, JC; Streib, WE; Wentworth, RAD Trigonal Prismatic Co-ordination in the cis,cis-1,3,5-Tris(pyridine-2 carboxaldimino)cyclohexanezinc(II) Ion J. Chem. Soc, 1969, (15), 843-844.].
Pioneering work of L. Pauling and R. G. Dickinson’s on the study of mineral molybdenite [5Dickinson, RG; Pauling, L The crystal structure of molybdenite J. Am. Chem. Soc, 1923, 45, 1466-1471.
[http://dx.doi.org/10.1021/ja01659a020] ], in which sulfur atoms surrounds the molybdenum in a trigonal prismatic coordination arrangement, initially invoked interest in the subject. Consequently, a range of analogous compounds were investigated and shown to exhibit TP or pseudo TP arrangements [6(a) Eisenberg, R; Ibers, JA Trigonal Prismatic Coordination. The Molecular Structure of Tris(cis-1,2-diphenylethene-1,2-dithiolato)rhenium J. Am. Chem. Soc, 1965, 87, 3776-3778.(b)Eisenberg, R; Ibers, JA Trigonal Prismatic Coordination. The Crystal and Molecular Structure of Tris(cis-1,2-diphenylethene-1,2-dithiolato)rhenium Inorg. Chem, 1966, 5, 411-416., 7(a) Eisenberg, R; Stiefel, EI; Rosenberg, RC Six-Coordinate Trigonal-Prismatic Complexes of First-Row Transition Metals J.
Am. Chem. Soc, 1966, 88, 2874-2876.
[http://dx.doi.org/10.1021/ja00964a061] (b) Eisenberg, R; Gray, HB Trigonal-prismatic coordination. Crystal and Molecular Structure of Tris (cis-1,2-diphenylethylene-1,2-dithiolato)vanadium Inorg. Chem, 1967, 6, 1844-1849.
[http://dx.doi.org/10.1021/ic50056a018] ]. Several other structurally rigid and non rigid ligand systems were also reported. The structurally rigid systems utilised a variety of ligands, such as the bimacro-cyclic ligands, bicapped-TRENCAM (BCT) and bicapped-TPTCAM (BCTPT) [8Karpishin, TB; Stack, TDP; Raymond, KN Octahedral versus trigonal prismatic geometry in a series of catechol macrobicyclic ligand-metal complexes J. Am. Chem. Soc, 1993, 115, 182-192.
[http://dx.doi.org/10.1021/ja00054a025] ], N,N,N’,N’-tetramethyle-thylenediamine (tmeda)[9Morse, PM; Girolami, GS Are d0 ML6 complexes always octahedral? The x-ray structure of trigonal-prismatic [Li(tmed)]2[ZrMe6] J. Am. Chem. Soc, 1989, 111, 4114-4116.
[http://dx.doi.org/10.1021/ja00193a061] ], (py)3tach, (py)3tame and clathro-chelates [10(a) Parks, JE; Wagner, BE; Holm, RH Three-dimensional macrocyclic encapsulation reactions. I. Synthesis of six-coordinate complexes with nonoctahedral stereochemistry J. Am. Chem. Soc, 1970, 92, 3500-3502.(b) Parks, JE; Wagner, BE; Holm, RH Three-dimensional macrocyclic encapsulation reactions. II. Synthesis and properties of nonoctahedral clathro chelates derived from tris(2-aldoximo-6-pyridyl)phosphine and boron trifluoride or tetrafluoroborate Inorg. Chem, 1971, 10, 2472-2478.
[http://dx.doi.org/10.1021/ic50105a021] (c) Churchill, MR; Reis, AH Structural studies on clathro chelate complexes. I.
Trigonal-prismatic coordination of d8 nickel(II) in crystalline
[fluoroborotris(2-aldoximo-6-pyridyl)phosphine]nickel(II) tetrafluoroborate Inorg. Chem, 1972, 11, 1811-1818.
[http://dx.doi.org/10.1021/ic50114a014] ], to name but a few. More recently, tris(pyrazolyl)borate (Tppy) based ligand systems were coordinated with Co (II) by Ward and McCleverty et al. [11Paul, RL; Amoroso, AJ; Jones, PL; Couchman, SM; Reeves, ZR; Rees, LH; Jeffery, JC; McCleverty, JA; Ward, MD Effects of metal co-ordination geometry on self-assembly: a monomeric complex with trigonal prismatic metal co-ordination vs. tetrameric complexes with octahedral metal co-ordination J. Chem. Soc, 1999, 28(10), 1563-1568.], and the trigonal prismatic conformation was observed, however other metal ions yielded tetrameric complexes containing octahedrally co-ordinated metals.
Recently, we have investigated the metal driven self assembly of a six-coordinate tripodal ligand, tris(2,2’-bipyrid-6-yl)methanol with series of transition metals [12Knight, JC; Alvarez, S; Amoroso, AJ; Edwards, PG; Singh, N A novel bipyridine-based hexadentate tripodal framework with a strong preference for trigonal prismatic co-ordination geometries Dalton. Trans, 2010, 39(16), 3870-3883.
[http://dx.doi.org/10.1039/b924651g] ]. Furthermore, we have focussed our attention in investigating the relationship between octahedral and trigonal prismatic character while varying d electron configuration. It was established that unlike the monoanionic TpPy, the bipy based ligand naturally favours monomeric complexes as the formation of tetrameric complexes will induce unfavourable electrostatic repulsion. It was concluded that configurationally restrained ligand framework was responsible for enforcing this comparatively rare geometry with respect to an octahedron. In this work we wished to investigate the solid state and solution state structure of the ligand without a metal ion co-ordinated to it. Single crystals of this ligand have been isolated for the first time in the protonated form as a perchlorate salt. We have determined the structure of this species, as well as obtaining the 1H NMR spectra of the ligand and its protonated form. These finding will be discussed below.
EXPERIMENTAL SECTION
All materials were of reagent grade and used as received from commercial sources. Solvents were distilled before use. Ligand L1 (Fig. 1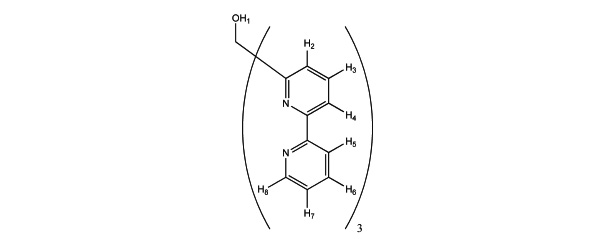 ) was prepared as described in our previous work [12Knight, JC; Alvarez, S; Amoroso, AJ; Edwards, PG; Singh, N A novel bipyridine-based hexadentate tripodal framework with a strong preference for trigonal prismatic co-ordination geometries Dalton. Trans, 2010, 39(16), 3870-3883.
) was prepared as described in our previous work [12Knight, JC; Alvarez, S; Amoroso, AJ; Edwards, PG; Singh, N A novel bipyridine-based hexadentate tripodal framework with a strong preference for trigonal prismatic co-ordination geometries Dalton. Trans, 2010, 39(16), 3870-3883.
[http://dx.doi.org/10.1039/b924651g] ]. L1 (50 mg, 0.10 mmol) was dissolved in methanol (15 mL) and then added dropwise to an aqueous solution of praseodymium(III) perchlorate (110 mg, 101 mmol; 40 wt % aqueous solution). The solution was stirred for 20 minutes and left at 4°C for 2 days. A light brown precipitate was filtered off and the remaining filtrate was left to slowly evaporate at room temperature, yielding rectangular shaped crystals suitable for X-ray diffraction (30%). 1H NMR (400 MHz; CD3CN): 8.63 (d, 3H, J = 8.64 Hz, py-H8), 8.40 (d, 3H, J = 8.53 Hz, py-H4), 8.35 (d, 3H, J = 8.5 Hz, py-H5), 8.13 (t, 3H, J = 8.38 Hz, py-H3), 8.05 (d, 3H, J = 8.35 Hz, py-H2), 8.00 (t, 3H, J = 8.32 Hz, py-H6), 7.55 ( t, 3H, J = 8.09 Hz, py-H7).
 |
Fig. (1) Ligand L 1 showing the proton numbering. |
X-RAY CRYSTALLOGRAPHY
All single crystal X-ray data was collected at 150 K on a Bruker/Nonius Kappa CCD diffractometer using graphite monochromatic Mo-Kα radiation (λ = 0.71073 Å), equipped with an Oxford Cryostream cooling apparatus. Crystal parameters and details of the data collection, solution and refinement are presented in Table 1. The data was corrected for Lorentz and polarisation effects and for absorption using SORTAV [13Blessing, RH An empirical correction for absorption anisotropy Acta. Crystallogr. A, 1995, 51, 33-38.
[http://dx.doi.org/10.1107/S0108767394005726] ]. Structure solution was achieved by direct methods (Sir- 92 program system [14Altomare, A; Cascarano, G; Giacovazzo, C; Guagliardi, A Completion and refinement of crystal structures with SIR92 J. Appl. Crystallogr, 1993, 26, 343-350.
[http://dx.doi.org/10.1107/S0021889892010331] ] and refined by full-matrix least-squares on F2 (SHELXL-97 [15Sheldrick, GM SHELXL-97: Program for the Refinement of Crystal Structures University of Göttingen: Göttingen, Germany, 1997. ] with all non-hydrogen atoms assigned anisotropic displacement parameters. Hydrogen atoms attached to carbon atoms were placed in idealised positions and allowed to ride on the relevant carbon atom. In the final cycles of refinement, a weighting scheme that gave a relatively flat analysis of variance was introduced and refinement continued until convergence was reached. Molecular structures in the figures were drawn with ORTEP 3.0 for Windows (version 1.08) [16Farrugia, LJ Ortep-3 for Windows - A Version of ORTEP-III with a Graphical User Interface (GUI) J. Appl. Crystallogr, 1997, 30, 565.
[http://dx.doi.org/10.1107/S0021889897003117] ]. Crystallographic data for 1 in CIF format have been deposited with the Cambridge Crystallographic Data Centre (CCDC 783585). Copies of these later data may be obtained free of charge via www.ccdc.cam.ac.uk/data_request/Cif, by emailing data_request@ccdc.cam.ac.uk, or by contacting the CCDC, 12 Union Road, Cambridge CB2 IEZ, UK; Fax: +44-1223-336033.
RESULTS AND DISCUSSION
The crystal structure of 1 is depicted in Fig. (2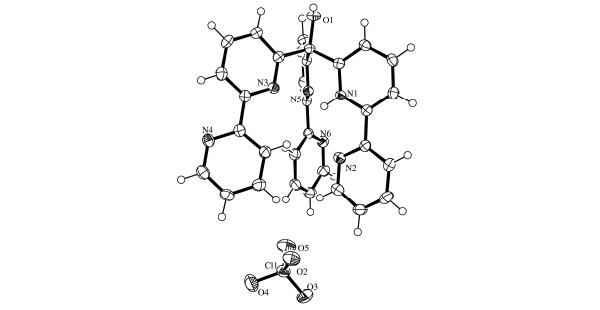 ). The ligand consists of three bipy groups linked via a methine bridge. One of these bipy groups is monoprotonated and is held in a cisoid configuration by an intramolecular hydrogen bond (Table 2). The dihedral angle between the least-squares planes of these two pyridyl units is just 9.27°. The two remaining bipy groups are not configurationally restrained by any such intramolecular interactions and each exhibits a transoid configuration around the interannular bond. Accordingly, the dihedral angle for the N3/N4-containing bipy group is 22.27° and for the N5/N6-containing bipy group this value is larger at 36.54°. The three bipy arms adopt an oblique configuration (Fig. 3
). The ligand consists of three bipy groups linked via a methine bridge. One of these bipy groups is monoprotonated and is held in a cisoid configuration by an intramolecular hydrogen bond (Table 2). The dihedral angle between the least-squares planes of these two pyridyl units is just 9.27°. The two remaining bipy groups are not configurationally restrained by any such intramolecular interactions and each exhibits a transoid configuration around the interannular bond. Accordingly, the dihedral angle for the N3/N4-containing bipy group is 22.27° and for the N5/N6-containing bipy group this value is larger at 36.54°. The three bipy arms adopt an oblique configuration (Fig. 3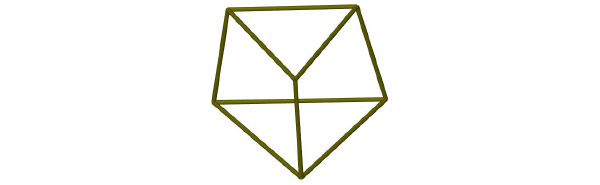 ), spreading further apart as the sterical influence conferred from the methine bridge is reduced. The C-N and mean C-C bond lengths for the protonated pyridine are 1.355(3) Å and 1.384(3) Å respectively. The corresponding values for the neutral pyridyl units are statistically identical at 1.344(3) Å and 1.385(3) Å. These bond lengths are in excellent agreement with literature values for both 2,2’-bipyridine [17Merritt, Jr LL; Schroeder, ED The Crystal Structure of 2,2'-Bipyridine Acta. Crystallogr, 1956, 9, 801-804.
), spreading further apart as the sterical influence conferred from the methine bridge is reduced. The C-N and mean C-C bond lengths for the protonated pyridine are 1.355(3) Å and 1.384(3) Å respectively. The corresponding values for the neutral pyridyl units are statistically identical at 1.344(3) Å and 1.385(3) Å. These bond lengths are in excellent agreement with literature values for both 2,2’-bipyridine [17Merritt, Jr LL; Schroeder, ED The Crystal Structure of 2,2'-Bipyridine Acta. Crystallogr, 1956, 9, 801-804.
[http://dx.doi.org/10.1107/S0365110X56002175] ] and 2,2’-bipyridinium (bis)perchlorate [18Ma, G; Ilyukhin, A; Glaser, J 2,2'-Bipyridinium bis(perchlorate) Acta. Crystallogr. C, 2000, 56, 1473-1475.
[http://dx.doi.org/10.1107/S0108270100012452] ].
Another feature of interest is the orientation of the pyridine rings directly bonded to the bridgehead carbon of the tripod (the proximal pyridine donors). The crystallographic data for tris(2-pyridyl)methanol [19Keene, FR; Snow, MR; Tiekink, ERT Tris(2-pyridyl)methanol Acta. Crystallogr. C, 1988, 44, 937-938.
[http://dx.doi.org/10.1107/S0108270188000411] ] shows these rings positioned such that all three lone pairs on the nitrogens are pointing away from each other.
However, when protonated, two rings interact with the proton, leading to a N-N distance of 2.656 Å [20Boggess, RK Monoprotonated perchlorate salt of tris(2-pyridyl)methanol Acta. Crystallogr. C, 1992, 48, 1327-1329.
[http://dx.doi.org/10.1107/S0108270191013860] ]. In contrast, protonated tris(2,2’-bipyrid-6-yl) methanol has all three lone pairs of the proximal pyridine donors pointing towards the proton. In addition, one distal pyridine is also hydrogen bonding to the proton. This distal pyridine (N2-C7/C11) is part of the bipyridyl unit to which the proton is most strongly associated. The N-N distances between the pyridinium moiety (N1-C2/C6) and the two other proximal pyridines is 2.786(3) Å (N1-N5) and 2.852(3) Å (N1-N3), while the remaining N-N distance between these proximal donors is 3.048(3) Å (N3-N5). The N-N distance between the proximal pyridinium unit and its distal pyridine is 2.667(3) Å (N1-N2).
The molecular packing is consolidated by various intermolecular interactions, such as π-stacking between N1-C2/C6 and N2-C7/C11 (symmetry code: 2-x, 1-y, -z; Fig. 4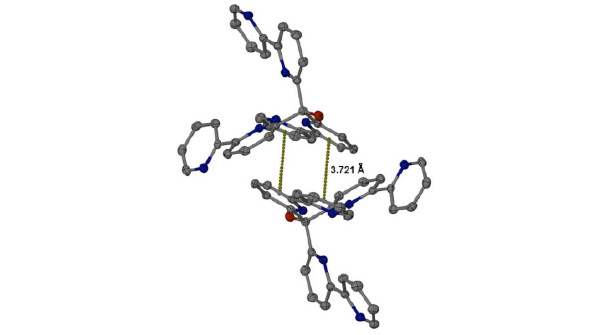 ) and several H-bonds involving the perchlorate counterion and three water molecules. The alcohol group (O1) is also acting as an H-bond donor to a water molecule (O8) within the lattice.
) and several H-bonds involving the perchlorate counterion and three water molecules. The alcohol group (O1) is also acting as an H-bond donor to a water molecule (O8) within the lattice.
The 1H NMR 2-D COSY spectra of both the mono-protonated and neutral forms of 1 were measured in order to elucidate the coupling behaviour within the aromatic region and thus unambiguously assign the resonances (Fig. 5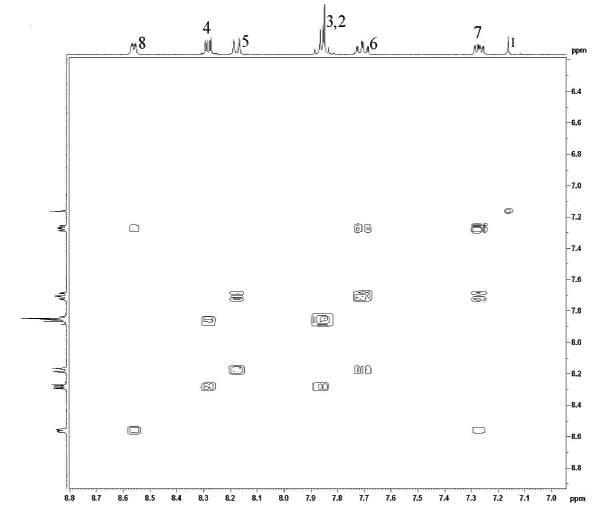 and Fig. 6
and Fig. 6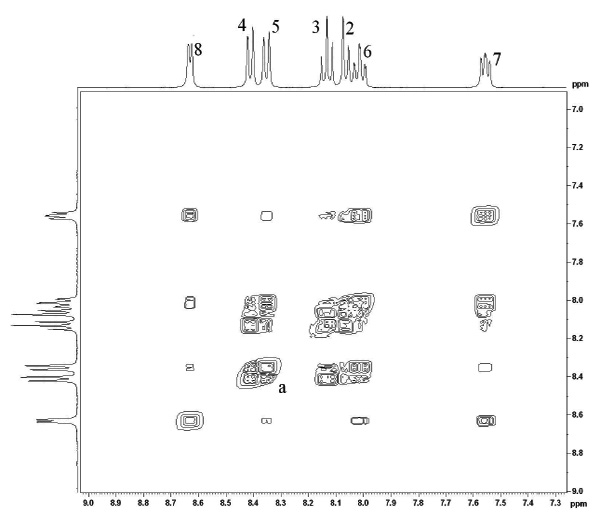 , respectively). First, one can note that the addition of a single proton to the tripod structure does not destroy the C3 symmetry of the molecule and all bipyridyl moieties are still equivalent. This would be consistent with fluxional behaviour of the proton, faster than the NMR timescale. Also, whilst the proton resonance frequencies for both the mono-protonated and neutral ligand exhibit similar ordering, those of the protonated form are shifted slightly downfield as one would expect from the deshielding effect of adding a proton to the pyridine ring [21Proton NMR data for the neutral ligand (400 MHz; CDCl3): δ 8.55 (d, 3H, J = 7.8 Hz), 8.28 (d, 3H, J = 7.5 Hz), 8.12 (d, 3H, J = 7.2
Hz), 7.89 – 7.72 (m, 6H), 7.67 (t, 3H, J = 7.5 Hz), 7.21 (t, 3H, J = 7.2 Hz), 5.21 (s, 1H), ].
, respectively). First, one can note that the addition of a single proton to the tripod structure does not destroy the C3 symmetry of the molecule and all bipyridyl moieties are still equivalent. This would be consistent with fluxional behaviour of the proton, faster than the NMR timescale. Also, whilst the proton resonance frequencies for both the mono-protonated and neutral ligand exhibit similar ordering, those of the protonated form are shifted slightly downfield as one would expect from the deshielding effect of adding a proton to the pyridine ring [21Proton NMR data for the neutral ligand (400 MHz; CDCl3): δ 8.55 (d, 3H, J = 7.8 Hz), 8.28 (d, 3H, J = 7.5 Hz), 8.12 (d, 3H, J = 7.2
Hz), 7.89 – 7.72 (m, 6H), 7.67 (t, 3H, J = 7.5 Hz), 7.21 (t, 3H, J = 7.2 Hz), 5.21 (s, 1H), ].
 |
Fig. (5) A COSY map showing the expanded aromatic region of the neutral unprotonated ligand taken at 293K in CD3CN. |
 |
Fig. (6) A COSY map showing the expanded aromatic region of the protonated ligand taken at 293K in CD3CN. |
In both cases, there exists strong coupling between vicinal protons around each of the pyridine groups. This allow us to unambiguously group the resonances for each pyridyl ring ((H2, H3, H4,) vs (H5, H6, H7, H8)), when considering the cross peaks. By then assigning the peak which the highest ppm to H8 (ortho to the nitrogen atom), this allows the full assignment of all peaks. This is particularly useful in discriminating between H4 and H5 as well as H3 and H6. This allows us to determine that the relative ordering of all resonances is unchanged between the protonated and free ligand and suggests there is no preferencial localisation of the proton on one of the two different pyridyl rings.
The protonated ligand also reveals evidence of long range coupling between the two pyridines, which are in a cisoid arrangement, specifically through H4 and H5 (labelled a).
CONCLUSION
In conclusion, we have shown that the solid state structure of the protonated form of the ligand L1 has a structure consisting of the three bipyridyl moieties connected to a central carbon. The pyridyl rings connected directly to the central carbon all having their nitrogen donor pointing to the centre of the molecule, perhaps suggesting they all interact with the proton. The other three nitrogen donors on the outermost pyridines vary in their behaviour, with two having a transoid arrangement within the bipyridine moieties and one being cis. However, this asymmetric arrangement is not observed in the solution state as 1H NMR identifies C3 symmetry in the complex. COSY NMR has allowed us to unambiguously assign the proton resonances. Interestingly, the similar ordering of the resonances, in the free ligand compared to its protonated form, suggests little difference in the relative electron density within a compound.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
We gratefully acknowledge the support of Cardiff University.
REFERENCES
| [1] | Whitesides, GM; Boncheva, M Beyond molecules: Self-assembly of mesoscopic and macroscopic components Proc. Natl. Acad. Sci.
U S A, 2002, 99, 4769-4774. [http://dx.doi.org/10.1073/pnas.082065899] |
| [2] | Hu, WJ; Liu, LQ; Ma, ML; Zhao, XL; Liu, YA; Mi, XQ; Jiang, B; Wen, K A Trigonal Prismatic Ligand in the Metal-Mediated Self-Assembly of One- and Two-Dimensional Metallosupramolecular Polymers Inorg. Chem, 2013, 52, 9309-9319. |
| [3] | Company, A; Roques, N; Güell, M; Mugnaini, V; Gómez, L; Imaz, I; Datcu, A; Solà, M; Luis, JM; Veciana, J; Ribas, X; Costas, M Nanosized trigonal prismatic and antiprismatic CuII coordination cages based on tricarboxylate linkers Dalton. Trans, 2008, 37(13), 1679-1682. |
| [4] | Gillum, WO; Huffman, JC; Streib, WE; Wentworth, RAD Trigonal Prismatic Co-ordination in the cis,cis-1,3,5-Tris(pyridine-2 carboxaldimino)cyclohexanezinc(II) Ion J. Chem. Soc, 1969, (15), 843-844. |
| [5] | Dickinson, RG; Pauling, L The crystal structure of molybdenite J. Am. Chem. Soc, 1923, 45, 1466-1471. [http://dx.doi.org/10.1021/ja01659a020] |
| [6] | (a) Eisenberg, R; Ibers, JA Trigonal Prismatic Coordination. The Molecular Structure of Tris(cis-1,2-diphenylethene-1,2-dithiolato)rhenium J. Am. Chem. Soc, 1965, 87, 3776-3778.(b)Eisenberg, R; Ibers, JA Trigonal Prismatic Coordination. The Crystal and Molecular Structure of Tris(cis-1,2-diphenylethene-1,2-dithiolato)rhenium Inorg. Chem, 1966, 5, 411-416. |
| [7] | (a) Eisenberg, R; Stiefel, EI; Rosenberg, RC Six-Coordinate Trigonal-Prismatic Complexes of First-Row Transition Metals J.
Am. Chem. Soc, 1966, 88, 2874-2876. [http://dx.doi.org/10.1021/ja00964a061] (b) Eisenberg, R; Gray, HB Trigonal-prismatic coordination. Crystal and Molecular Structure of Tris (cis-1,2-diphenylethylene-1,2-dithiolato)vanadium Inorg. Chem, 1967, 6, 1844-1849. [http://dx.doi.org/10.1021/ic50056a018] |
| [8] | Karpishin, TB; Stack, TDP; Raymond, KN Octahedral versus trigonal prismatic geometry in a series of catechol macrobicyclic ligand-metal complexes J. Am. Chem. Soc, 1993, 115, 182-192. [http://dx.doi.org/10.1021/ja00054a025] |
| [9] | Morse, PM; Girolami, GS Are d0 ML6 complexes always octahedral? The x-ray structure of trigonal-prismatic [Li(tmed)]2[ZrMe6] J. Am. Chem. Soc, 1989, 111, 4114-4116. [http://dx.doi.org/10.1021/ja00193a061] |
| [10] | (a) Parks, JE; Wagner, BE; Holm, RH Three-dimensional macrocyclic encapsulation reactions. I. Synthesis of six-coordinate complexes with nonoctahedral stereochemistry J. Am. Chem. Soc, 1970, 92, 3500-3502.(b) Parks, JE; Wagner, BE; Holm, RH Three-dimensional macrocyclic encapsulation reactions. II. Synthesis and properties of nonoctahedral clathro chelates derived from tris(2-aldoximo-6-pyridyl)phosphine and boron trifluoride or tetrafluoroborate Inorg. Chem, 1971, 10, 2472-2478. [http://dx.doi.org/10.1021/ic50105a021] (c) Churchill, MR; Reis, AH Structural studies on clathro chelate complexes. I. Trigonal-prismatic coordination of d8 nickel(II) in crystalline [fluoroborotris(2-aldoximo-6-pyridyl)phosphine]nickel(II) tetrafluoroborate Inorg. Chem, 1972, 11, 1811-1818. [http://dx.doi.org/10.1021/ic50114a014] |
| [11] | Paul, RL; Amoroso, AJ; Jones, PL; Couchman, SM; Reeves, ZR; Rees, LH; Jeffery, JC; McCleverty, JA; Ward, MD Effects of metal co-ordination geometry on self-assembly: a monomeric complex with trigonal prismatic metal co-ordination vs. tetrameric complexes with octahedral metal co-ordination J. Chem. Soc, 1999, 28(10), 1563-1568. |
| [12] | Knight, JC; Alvarez, S; Amoroso, AJ; Edwards, PG; Singh, N A novel bipyridine-based hexadentate tripodal framework with a strong preference for trigonal prismatic co-ordination geometries Dalton. Trans, 2010, 39(16), 3870-3883. [http://dx.doi.org/10.1039/b924651g] |
| [13] | Blessing, RH An empirical correction for absorption anisotropy Acta. Crystallogr. A, 1995, 51, 33-38. [http://dx.doi.org/10.1107/S0108767394005726] |
| [14] | Altomare, A; Cascarano, G; Giacovazzo, C; Guagliardi, A Completion and refinement of crystal structures with SIR92 J. Appl. Crystallogr, 1993, 26, 343-350. [http://dx.doi.org/10.1107/S0021889892010331] |
| [15] | Sheldrick, GM SHELXL-97: Program for the Refinement of Crystal Structures University of Göttingen: Göttingen, Germany, 1997. |
| [16] | Farrugia, LJ Ortep-3 for Windows - A Version of ORTEP-III with a Graphical User Interface (GUI) J. Appl. Crystallogr, 1997, 30, 565. [http://dx.doi.org/10.1107/S0021889897003117] |
| [17] | Merritt, Jr LL; Schroeder, ED The Crystal Structure of 2,2'-Bipyridine Acta. Crystallogr, 1956, 9, 801-804. [http://dx.doi.org/10.1107/S0365110X56002175] |
| [18] | Ma, G; Ilyukhin, A; Glaser, J 2,2'-Bipyridinium bis(perchlorate) Acta. Crystallogr. C, 2000, 56, 1473-1475. [http://dx.doi.org/10.1107/S0108270100012452] |
| [19] | Keene, FR; Snow, MR; Tiekink, ERT Tris(2-pyridyl)methanol Acta. Crystallogr. C, 1988, 44, 937-938. [http://dx.doi.org/10.1107/S0108270188000411] |
| [20] | Boggess, RK Monoprotonated perchlorate salt of tris(2-pyridyl)methanol Acta. Crystallogr. C, 1992, 48, 1327-1329. [http://dx.doi.org/10.1107/S0108270191013860] |
| [21] | Proton NMR data for the neutral ligand (400 MHz; CDCl3): δ 8.55 (d, 3H, J = 7.8 Hz), 8.28 (d, 3H, J = 7.5 Hz), 8.12 (d, 3H, J = 7.2 Hz), 7.89 – 7.72 (m, 6H), 7.67 (t, 3H, J = 7.5 Hz), 7.21 (t, 3H, J = 7.2 Hz), 5.21 (s, 1H), |