- Home
- About Journals
-
Information for Authors/ReviewersEditorial Policies
Publication Fee
Publication Cycle - Process Flowchart
Online Manuscript Submission and Tracking System
Publishing Ethics and Rectitude
Authorship
Author Benefits
Reviewer Guidelines
Guest Editor Guidelines
Peer Review Workflow
Quick Track Option
Copyediting Services
Bentham Open Membership
Bentham Open Advisory Board
Archiving Policies
Fabricating and Stating False Information
Post Publication Discussions and Corrections
Editorial Management
Advertise With Us
Funding Agencies
Rate List
Kudos
General FAQs
Special Fee Waivers and Discounts
- Contact
- Help
- About Us
- Search

Open Chemistry Journal
(Discontinued)
ISSN: 1874-8422 ― Volume 8, 2021
Crystal Structure Determination and Molecular Docking Studies of 4- (5-Phenyl Pyrazin-2-Yl)-4h-1,2,4 Triazole-3-Thiol with Focal Adhesion Kinase Inhibitors
K.S. Kiran1, 2, *, M.K. Kokila2, Guruprasad R3, Niranjan M.S4
Abstract
The main objective of the present work is to determine crystal structure of the ligand by x-ray methods and to perform molecular docking studies of the ligand 4- Phenyl -5-Pyrazinyl-3-mercapto 1,2,4 Triazole with protein focal adhesion kinase (FAK) domain using the software, Autodock and pymol. Macromolecular modeling by docking studies provides the most detailed view possible of drug receptor interaction. It has created a new rational approach to drug design, where the structure of drug is designed, based on its fit to three dimensional structures of receptor site, rather than basing it on analogies to other active structures. The above titled compound is binding with FAK protein. This may act as inhibitor to FAK and can be used for anticancer therapy target.
Article Information
Identifiers and Pagination:
Year: 2016Volume: 3
First Page: 69
Last Page: 74
Publisher Id: CHEM-3-69
DOI: 10.2174/1874842201603010069
Article History:
Received Date: 23/4/2015Revision Received Date: 8/7/2016
Acceptance Date: 27/7/2016
Electronic publication date: 15/09/2016
Collection year: 2016
open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution-Non-Commercial 4.0 International Public License (CC BY-NC 4.0) (https://creativecommons.org/licenses/by-nc/4.0/legalcode), which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
* Address correspondence to this author at the Department of Physics, School of Engineering and Technology, Jain University, Bangalore, India; Tel: +9448419437; E-mail: kiranxrd@gmail.com
| Open Peer Review Details | |||
|---|---|---|---|
| Manuscript submitted on 23-4-2015 |
Original Manuscript | Crystal Structure Determination and Molecular Docking Studies of 4- (5-Phenyl Pyrazin-2-Yl)-4h-1,2,4 Triazole-3-Thiol with Focal Adhesion Kinase Inhibitors | |
INTRODUCTION
1,2,4 Triazole is one of the organic heterocyclic compounds containing a five membered di-unsaturated ring structure compound with three nitrogen atoms and two carbon atoms at nonadjacent positions. Although most triazoles are readily prepared and stored. 1,2,4-Triazole is a basic aromatic heterocycles and one of a pair of isomeric chemical compounds with molecular formula C2H3N3. This compound is screened for its anti cancerous, antibacterial and anti-microbial activity [1Mathew, V.; Keshavayya, J.; Vaidya, V.P. Heterocyclic system containing bridgehead nitrogen atom: synthesis and pharmacological activities of some substituted 1,2,4-triazolo[3,4-b]-1,3,4-thiadiazoles. Eur. J. Med. Chem., 2006, 41(9), 1048-1058.
[http://dx.doi.org/10.1016/j.ejmech.2006.03.018] [PMID: 16822595] -3Purohit, M.N.; Pujar, G.; Manohar, K.V.; Udupi, R.H.; Vijayakumar, G.S. Synthesis and antimicrobial activity of N-N- Bis{3-subsituted-5-mercapto1,2,4 triazole-4-yl}butane1,4-dicarboxamide derivatives. Indian J. Heterocycl. Chem., 2006, 16, 93-94.]. The molecular docking studies of 4- Phenyl -5-Pyrazinyl-3-mercapto 1,2,4 triazole with protein FAK [4André, E.; Becker-André, M. Expression of an N-terminally truncated form of human focal adhesion kinase in brain. Biochem. Biophys. Res. Commun., 1993, 190(1), 140-147.
[http://dx.doi.org/10.1006/bbrc.1993.1022] [PMID: 8422239] ] were performed by using Autodock. Docking studies have become nearly indispensable for the study of macromolecular structures and interactions.
MATERIALS AND METHODS
Database and Software
Data collection and cell refinement: CAD-4 software; Data reduction: MOLEN, Structure solution and refinement: SHELX97, Molecular graphics: ORTEP and PLATON Material for publication: SHELX97, program used for docking: Autodock [5Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem., 2009, 30(16), 2785-2791.
[http://dx.doi.org/10.1002/jcc.21256] [PMID: 19399780] ] and Pymol, File format conversion of the coordinates: openbabel.
PREPARATION OF LIGAND STRUCTURE
The crystals of the compound 4- Phenyl -5-Pyrazinyl-3-mercapto 1,2,4 triazole were grown by the slow evaporation technique using ethanol as solvent. The x-ray intensity data of the crystals was collected on a Bruker smart CCD diffractometer graphite monochromatic Mokα radiation. From the diffraction data,it was seen that the title molecule C12H9N5S crystallizes in monoclinic space group P21/c with unit cell dimensions, a=9.630(4) Å b=15.023(6) Å c=9.022 (4) Å and Z=4. The ortep diagram [6Farrugia, L.J. ORTEP3 for Windows- A version of OrtepIII with a graphical user interface. J. Appl. Cryst., 1997, 30, 565.
[http://dx.doi.org/10.1107/S0021889897003117] , 7Farrugia, L.J. WinGX suite for small-molecule single-crystal crystallography. J. Appl. Cryst., 1999, 32, 837-838.
[http://dx.doi.org/10.1107/S0021889899006020] ] of the ligand showing 50% probability displacement ellipsoids is shown in
Fig. (1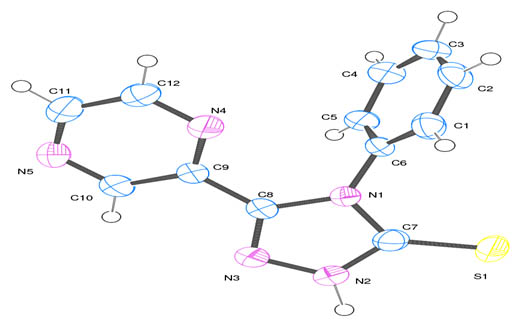 ). The crystal data of the compound are shown in Table 1.
). The crystal data of the compound are shown in Table 1.
 |
Fig. (1) Ortep diagram of 4- Phenyl -5-Pyrazinyl-3-mercapto 1,2,4 triazole. |
PREPARATION OF PROTEIN STRUCTURE
The 3D structure of FAK (PDB code: 2ETM) [8Andersen, C.B.; Ng, K.; Vu, C.; Ficarro, S.; Choi, H.-S.; Gray, N.; He, Y.; Lee, C.C. Crystal Structure of Focal Adhesion Kinase Domain Complexed with ATP and novel 7H- Pyrrolo [2,3-d] pyrimidine scaffolds. (to be Published).] was downloaded from the protein data bank. By adding the polar hydrogen bond to the receptor FAK which is required to convert PDB to PDBQT format. The water molecules around the ligand and cofactors were removed from the protein.
PROTEIN LIGAND INTERACTION
Docking allows the user to virtually screen a database of compounds and predict the strongest binders based on various scoring functions. The docking analysis of the ligand 4-Phenyl-5-pyrazinyl-3-mercapto-1,2,4 triazole (PYT3) and their analogs with focal adhesion kinase receptor was carried by using Autodock 4.2 and FlexX docking software. The reference ligand 7PY (7-Pyridin-2-Yl-N-(3,4,5-Trimethoxyphenyl)-7h-Pyrrolo[2,3-D] pyrimidin-2-Amine) was selected for the binding site analysis [9Rand, H.P.; Dale, M.M.; Ritter, M.M.; Moore, P.K. Pharmocology, 5th ed.; NewYork, 2003, pp. 51-63., 10Golubovskaya, V.M.; Kweh, F.A.; Cance, W.G. Focal adhesion kinase and cancer. Histol. Histopathol., 2009, 24(4), 503-510.
[PMID: 19224453] ]. Docking of the protein ligand complex was mainly targeted at the predicted active site. The interaction was carried out to find the favorable binding geometries of the ligand with the protein. The selected residues of the receptor were defined to be part to the binding site. The molecules binding to a receptor, ideally must inhibit its function, and thus act as drug [11Srivastava, V.; Kumar, A.; Mishra, B.N.; Siddiqi, M.I. Molecular docking studies on DMDP derivatives as human DHFR inhibitors. Bioinformation, 2008, 3(4), 180-188.
[http://dx.doi.org/10.6026/97320630003180] [PMID: 19238244] ]. The collection of 1,2,4 triazole derivatives and receptor complexes was identified via docking. Molecular docking was carried out to find the favorable binding geometries of the ligand with the protein. 1,2,4 triazole derivatives were then docked at predicted active site of FAK, using Autodock4.2 (Lemarkian Genetic Algorithm) and Flexx (Incremental Docking Algorithm). The pose received with the highest dock score from Flexx as well as lowest binding free energy from Autodock4 was then selected for further analysis [12Kramer, B.; Rarey, M.; Lengauer, T. Evaluation of the FLEXX incremental construction algorithm for protein-ligand docking. Proteins, 1999, 37(2), 228-241.
[http://dx.doi.org/10.1002/(SICI)1097-0134(19991101)37:2<228::AID-PROT8>3.0.CO;2-8] [PMID: 10584068] ]. The best binding pose at the binding site is shown in Figs. (2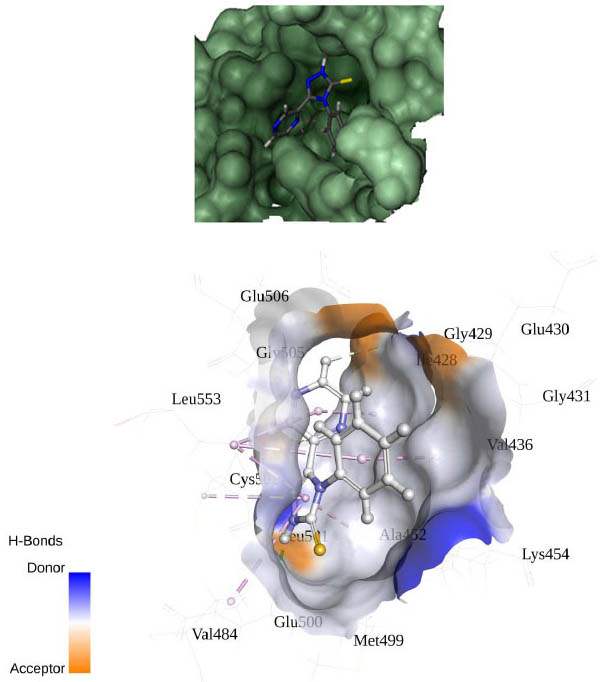 -4
-4 ). Table 2 shows different binding energies for different conformations of the ligand.
). Table 2 shows different binding energies for different conformations of the ligand.
 |
Fig. (2) Binding pose of ligand in binding site using flex. |
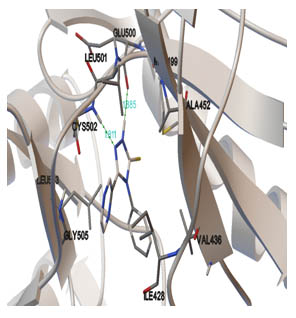 |
Fig. (3) Binding pose of ligand in binding site using Autodock. |
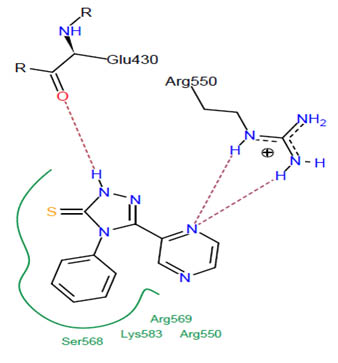 |
Fig. (4) The pharmacophore of the protein interacting with the ligand; residues forming hydrogen bonding. |
RESULTS AND DISCUSSION
The docking result showed the best 10 binding sites. The best dock energy -5.7 kcal/mol was campared with the reference ligand (7PY) and the docked energy was -5.87 kcal/mol, which showed that the protein ligand interactions are good fit with the target protein. The molecular docking studies shows good affinity of the ligand towards the protein. The higher the negative value of the energy of binding, the better is the affinity of the molecule to the receptor. The intermolecular interactions between the ligand and the protein were investigated. 1,2,4 Triazole for the most part, subsists in the 1H-1,2,4 triazole tautomeric form and the 4H-1,2,4 triazole form does not exist nor in solid state neither in solution form. In general amino substituted 1,2,4 triazoles were electronically preferred in a tautomeric equilibrium dominantly in 1H form [13Dolzhenko, A.V.; Pastorin, G.; Chui, W.K. An aqueous medium synthesis and tautomerism study of 3(5)-amino-1,2.4 Triazoles. Tetrahedron Lett., 2009, 50, 2124-2128.
[http://dx.doi.org/10.1016/j.tetlet.2009.02.172] ]. In the crystal lattice there were two intermolecular interactions, one potentially weak intramolecular and some C-π....π super molecular interactions [14Jeffrey, G.A.; Maluszynska, H.; Mitra, J. Hydrogen bonding in nucleosides and nucleotides. Int. J. Biol. Macromol., 1985, 7, 336-348.
[http://dx.doi.org/10.1016/0141-8130(85)90048-0] ]. In the solid state, supermolecular interactions stabilize the crystal structures.
CONCLUSION
The intermolecular interactions between the ligand and the protein were investigated. The synthesized chemical compound showed a good fit with the protein. Thus the bioactive compound interacting with the target can be used as a potent inhibitor to block the action of FAK protein. The docked value can be considered for further analysis.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
The author would like to thank to Prof.T.N.Guru Row, SSCU, IISC, Bangalore for providing the XRD facility. The author also thanks to Director, SET, Jain University, Bangalore for support and encouragement for my work.
REFERENCES
| [1] | Mathew, V.; Keshavayya, J.; Vaidya, V.P. Heterocyclic system containing bridgehead nitrogen atom: synthesis and pharmacological activities of some substituted 1,2,4-triazolo[3,4-b]-1,3,4-thiadiazoles. Eur. J. Med. Chem., 2006, 41(9), 1048-1058. [http://dx.doi.org/10.1016/j.ejmech.2006.03.018] [PMID: 16822595] |
| [2] | John, H.K.; John, M.B. Wilson and Gisvold's; Text Book of Organic Medical and Pharmaceutical Chemistry, 11th ed.; Lippincott Williams and Wilkins: Philadelphia, 2004, pp. 1-4. |
| [3] | Purohit, M.N.; Pujar, G.; Manohar, K.V.; Udupi, R.H.; Vijayakumar, G.S. Synthesis and antimicrobial activity of N-N- Bis{3-subsituted-5-mercapto1,2,4 triazole-4-yl}butane1,4-dicarboxamide derivatives. Indian J. Heterocycl. Chem., 2006, 16, 93-94. |
| [4] | André, E.; Becker-André, M. Expression of an N-terminally truncated form of human focal adhesion kinase in brain. Biochem. Biophys. Res. Commun., 1993, 190(1), 140-147. [http://dx.doi.org/10.1006/bbrc.1993.1022] [PMID: 8422239] |
| [5] | Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem., 2009, 30(16), 2785-2791. [http://dx.doi.org/10.1002/jcc.21256] [PMID: 19399780] |
| [6] | Farrugia, L.J. ORTEP3 for Windows- A version of OrtepIII with a graphical user interface. J. Appl. Cryst., 1997, 30, 565. [http://dx.doi.org/10.1107/S0021889897003117] |
| [7] | Farrugia, L.J. WinGX suite for small-molecule single-crystal crystallography. J. Appl. Cryst., 1999, 32, 837-838. [http://dx.doi.org/10.1107/S0021889899006020] |
| [8] | Andersen, C.B.; Ng, K.; Vu, C.; Ficarro, S.; Choi, H.-S.; Gray, N.; He, Y.; Lee, C.C. Crystal Structure of Focal Adhesion Kinase Domain Complexed with ATP and novel 7H- Pyrrolo [2,3-d] pyrimidine scaffolds. (to be Published). |
| [9] | Rand, H.P.; Dale, M.M.; Ritter, M.M.; Moore, P.K. Pharmocology, 5th ed.; NewYork, 2003, pp. 51-63. |
| [10] | Golubovskaya, V.M.; Kweh, F.A.; Cance, W.G. Focal adhesion kinase and cancer. Histol. Histopathol., 2009, 24(4), 503-510. [PMID: 19224453] |
| [11] | Srivastava, V.; Kumar, A.; Mishra, B.N.; Siddiqi, M.I. Molecular docking studies on DMDP derivatives as human DHFR inhibitors. Bioinformation, 2008, 3(4), 180-188. [http://dx.doi.org/10.6026/97320630003180] [PMID: 19238244] |
| [12] | Kramer, B.; Rarey, M.; Lengauer, T. Evaluation of the FLEXX incremental construction algorithm for protein-ligand docking. Proteins, 1999, 37(2), 228-241. [http://dx.doi.org/10.1002/(SICI)1097-0134(19991101)37:2<228::AID-PROT8>3.0.CO;2-8] [PMID: 10584068] |
| [13] | Dolzhenko, A.V.; Pastorin, G.; Chui, W.K. An aqueous medium synthesis and tautomerism study of 3(5)-amino-1,2.4 Triazoles. Tetrahedron Lett., 2009, 50, 2124-2128. [http://dx.doi.org/10.1016/j.tetlet.2009.02.172] |
| [14] | Jeffrey, G.A.; Maluszynska, H.; Mitra, J. Hydrogen bonding in nucleosides and nucleotides. Int. J. Biol. Macromol., 1985, 7, 336-348. [http://dx.doi.org/10.1016/0141-8130(85)90048-0] |